17
I F U
This product complies with the
requirements of the Medical Device
Regulation 2017/745
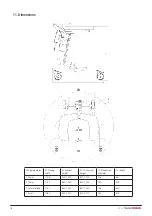
Visual Inspection
Medical Device
Read the manual
Caution
Product Code
Legal manufacturer
Direct Healthcare Group
Sverige AB
Batch Code
Type BF applied parts, according to the
degree of protection against electric shock
The device is intended for indoor use
WEEE Symbol
May not be discarded in domestic waste
Class II Equipment
Duty Cycle:
1 min in active (ON) mode.
19 min in rest (OFF) mode.
Maximum patient weight
Walker Height
Walker Width
Do not step on the device
Refer to instruction manual (IFU)
273
33
240
Mass
Weight (mass) of the device, the Maximum Patient Weight,
and total sum. All in kg.
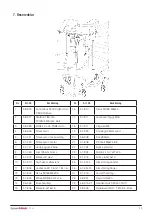
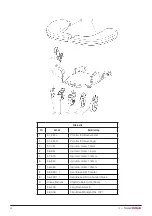
9. Table of Symbols
10. How to report a serious incident
Any serious incident that has occurred in relation to the device should be reported to the manufacturer and the
MHRA or another competent authority of the country in which the user and/or patient is established.
UK
[email protected]
T: +44 (0) 800 043 0881
F: +44 (0) 845 459 9832
Other:
[email protected]
Содержание SystemRoMedic 56-304
Страница 2: ...Table of contents English 4 Svenska 19 ...