Aztec 600 ISE ammonia and fluoride
Single-stream ion-selective analyzers
Appendix C – Principle of Operation – Ammonia Analyzers
OI/AXM630–EN Rev. I
81
Appendix C – Principle of Operation – Ammonia Analyzers
C.1 General Operation
The analyzer uses an ammonia probe. The probe contains a
glass pH electrode (whose pH-sensitive glass membrane forms
a slightly convex tip) and a robust, long-life reference electrode.
The two electrodes are combined into a single assembly and are
connected as a pH measuring pair through an internal reservoir
of filling solution. The filling solution is 0.1 M ammonium chloride
saturated with silver chloride and is separated from the sample
by a gas-permeable hydrophobic membrane fitted to the tip of
the probe.
As sample flows past the probe membrane, the partial
pressures of the ammonia gas in the sample on one side of the
membrane and the filling solution on the other equalize,
transferring gas across the membrane. At equal pressure, the
concentration of ammonia in the thin film of filling solution
between the probe membrane and the pH-sensitive glass
electrode membrane equals that in the sample. The resultant
change in the pH value of the thin film is measured by the pH
electrode pair, creating an output potential related to the
ammonia concentration in the sample. Like most ion-selective
electrodes, the ammonia probe produces an output that is
logarithmic in respect to concentration.
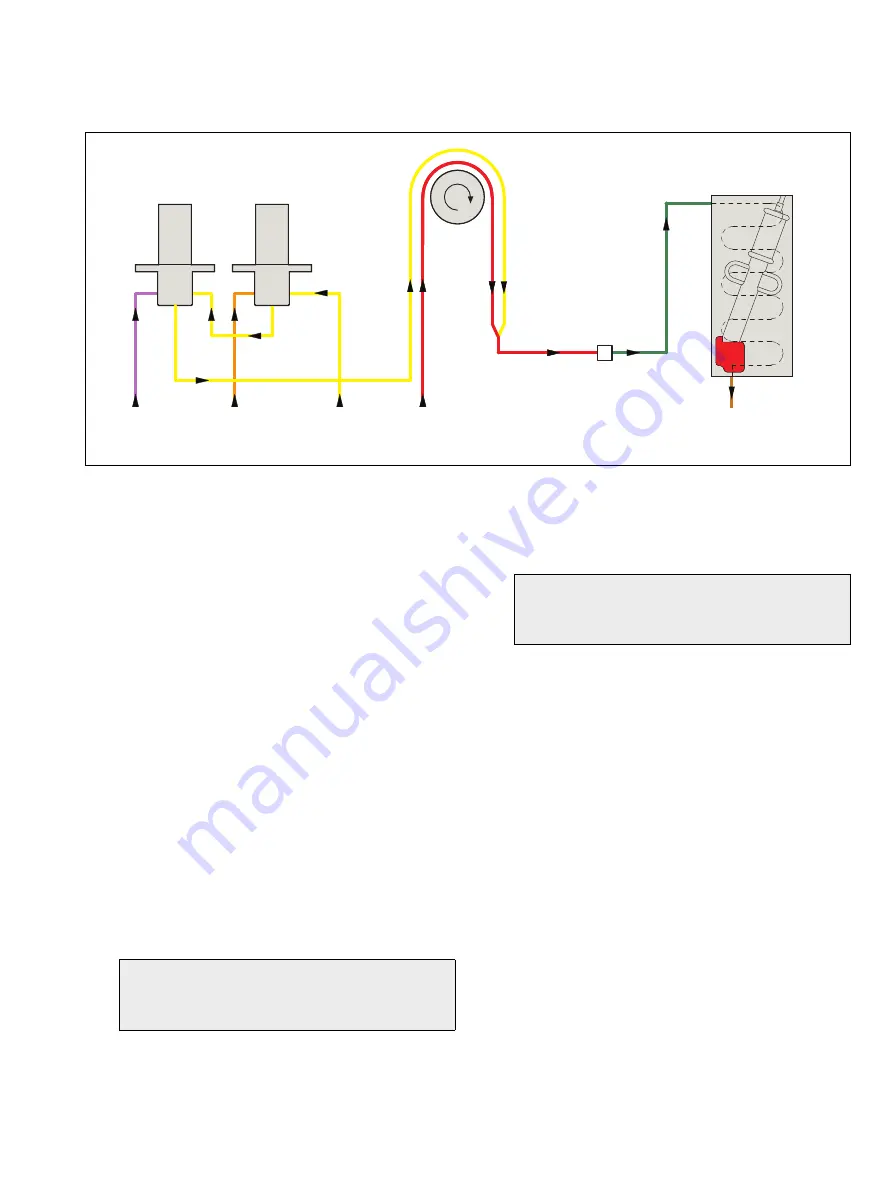
The analyzer's sequence of operation is as follows:
1. Sample is supplied to a side sample pot constant head
unit by a sample pump. Excess sample overflows to drain.
2. From the constant head unit, the sample is drawn through
the normally open ports of solenoid valves SV1 and SV2
by one channel of a peristaltic pump.
3. Reagent is drawn through another channel of the
peristaltic pump and mixed with the sample.
4. The conditioned sample is pumped into a flowcell
containing the ammonia probe. The probe is enclosed in a
heated block housing a heat-exchanger, ensuring that the
body of the probe remains at a constant temperature and
also at a temperature in agreement with that of the sample
and standard solutions.
5. When exposed to the reacted sample, the probe
produces an electrical potential that changes in proportion
to the changes in activity of the measured ion.
6. The probe is connected to the electronics section where,
after digital conversion, the signal is processed by
microprocessor.
7. After measurement, the sample flows to waste via a
contaminated drain connection.
During calibration, Low and High standard solutions are
introduced sequentially in place of the sample by means of
solenoid valves SV1 and SV2. The resultant millivolt Low and
millivolt High readings from the probe are stored as the
calibrated readings. The analyzer can be configured to perform
automatic calibrations from every 6 hours to once per week. A
calibration can also be initiated manually if required.
Fig. C.1 Flow Schematic
Waste
Standard
solution (low)
Standard
solution (high)
Reagent
Sample from
side sample pot
Reagent
pump
Ammonia flowcell
assembly (red)
Solenoid valve and manifold assemblies
C
o
nd
iti
o
ned samp
le
Note.
The constant head unit is fitted with a float
switch that signals an 'Out-of-sample' condition by
triggering an 'Out-of-Sample' alarm.
Note.
The sample and reagent tube diameters are
sized to obtain the correct ratio of sample and
reagent.
















































