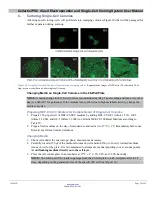
Cellartis iPSC rCas9 Electroporation and Single-Cell Cloning System User Manual
(030619)
takarabio.com
Takara Bio USA, Inc.
Page 21 of 24
2. Preparing DEF-CS SCC Medium for Single-Cell Seeding
Prepare the appropriate volume of DEF-CS SCC medium by adding DEF-CS GF-1 (dilute 1:333), GF-2
(dilute 1:1,000), and GF-3 (dilute 1:1,000) to Cellartis DEF-CS 500 Basal Medium according to Table VI.
Prepare fresh medium on the day of intended use and warm it to 37°C ± 1°C immediately before use.
Discard any leftover warmed medium.
It is important to prepare enough DEF-CS SCC medium to 1) neutralize the TrypLE Select Enzyme (1X)
used to dissociate cells from the initial culture vessel (a 1:10 dilution) and 2) seed the detached cells using
100 µl/well into a 96-well plate. Use Table VI as a guide to ensure there is sufficient medium for
dissociation and seeding.
Table VI. Preparation of medium for dissociation and single-cell seeding.
Cell Dissociation
Plate type
TrypLE Select Enzyme
(1X) (µl per well)
DEF-CS SCC medium
(µl per well)
48 wells
18
200
24 wells
38
400
12 wells
76
800
6 wells
190
2,000
10 cm
1,100
12,000
Single-Cell Seeding
Plate type
DEF-CS SCC medium (µl per well)
96 wells
100
3. Single-Cell Seeding
Seeding of Single Cells (Day 1)
1.
Check cells under a phase contrast microscope; photo document as necessary.
2.
Aspirate the medium from the culture vessel and wash the cell layer once with D-PBS –/–.
3.
Add TrypLE Select Enzyme (1X) to the culture vessel, using the amount indicated in Table VI. Place
the vessel in an incubator at 37°C ± 1°C, 5% CO
2
, and >90% humidity for 5 min, or until the cell
layer has detached. Detachment can be aided by tapping the side of the vessel firmly but gently. It is
not recommended to tilt or swirl the cell culture vessel.
NOTE:
If starting from multiple samples in the same plate, please make sure to replace the culture
vessel lid after removing a sample from a well, then gently tap the side of the vessel. This
redistributes the dissociation enzyme and minimizes the risk of the other samples drying out.
4.
Resuspend the cells in DEF-CS SCC medium (using the volume indicated in Table VI) and pipet up
and down several times to ensure a single-cell suspension. (The cells will aggregate if left too long in
TrypLE enzyme.)
5.
Use your preferred method to isolate single cells: FACS or limiting dilution. For limiting dilution, we
recommend using a final dilution of 0.5 cells per well of a 96-well plate.
6.
Place the plate in an incubator at 37°C ± 1°C, 5% CO
2
, and >90% humidity and leave the plate
undisturbed for 48 hr. Continue culturing according to Table IV.
Adding Media to Wells Containing Single Cells (Day 3)
Without discarding any medium, carefully add 100 µl of fresh DEF-CS SCC medium per well.
(See
Table IV for guidelines.) There should now be a total of 200 µl per well.