Study Endpoints
The primary and secondary endpoints were evaluated after 6 months of patient follow-up.
The primary safety endpoints were 1) Freedom from device / system-related
complications (DSRC), and 2) Freedom from pressure sensor failures. The primary
efficacy endpoint was the rate of HF hospitalizations. All hospitalizations were
adjudicated by an independent Clinical Events Committee (CEC) who were blinded to
treatment assignment. Secondary endpoints were tested in a hierarchical fashion and
included changes in PA pressures, proportion of subjects hospitalized for HF, days alive
outside of the hospital for HF, and quality of life. Because blinded follow-up continued
until the last patient completed 6 months of follow-up, the average patient follow-up was
much longer (17.6 months) and pre-specified supplementary analyses were conducted on
the full duration of follow-up data (Randomized Access).
Patient Demographics and Disposition
575 patients were consented for trial enrollment and underwent right heart catheterization.
Of these 575 patients, 25 (4.3%) underwent a right heart catheterization but did not
receive an implant primarily because of anatomical/physiological conditions identified
during the RHC. Of the 550 randomized patients, 270 were assigned to the Treatment
group and 280 to the Control group. The two groups were similar with respect to baseline
characteristics (Table 5).
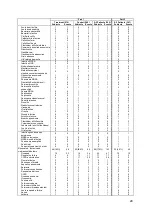
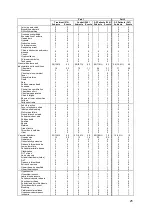
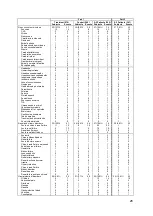
Table 5. Patient Demographics
Randomized Group
Variables
Treatment
(N=270)
Control
(N=280)
p-value
[1]
Age (years)
61.3 ± 12.98 (270) 61.8 ± 12.73 (280)
0.5927
Male
194/270 (71.9%)
205/280 (73.2%)
0.7745
Race (White)
196/270 (72.6%)
205/280 (73.2%)
0.9236
Systolic BP (mmHg)
121.2 ± 22.52 (270) 123.2 ± 21.01 (280)
0.1286
Heart Rate (bpm)
72.4 ± 12.91 (269) 73.0 ± 12.14 (280)
0.4873
BMI
30.5 ± 6.50 (270)
30.9 ± 7.35 (280)
0.6228
BUN (mg/dL)
29.6 ± 17.99 (248) 28.1 ± 16.17 (267)
0.6325
Creatinine (mg/dL)
1.4 ± 0.47 (270)
1.4 ± 0.42 (280)
0.5560
GFR (mL/min/1.73m²)
60.4 ± 22.50 (270) 61.8 ± 23.20 (280)
0.5638
Ejection Fraction (EF>=40%)
62/270 (23.0%)
57/279 (20.4%)
0.5343
Cardiac Output (L/min)
4.5 ± 1.41 (270)
4.6 ± 1.54 (278)
0.5499
Cardiac Index (L/min/m²)
2.1 ± 0.59 (270)
2.2 ± 0.64 (278)
0.4405
PVR
2.9 ± 2.02 (270)
2.7 ± 1.82 (278)
0.4609
PA Wedge Pressure (mmHg) 17.5 ± 7.97 (270)
19.0 ± 8.12 (280)
0.0276
PA Mean Pressure (mmHg)
28.9 ± 9.92 (270) 29.9 ± 10.05 (280)
0.3021
CRT-D/ICD Implant
179/270 (66.3%)
197/280 (70.4%)
0.3145
Ischemic Cardiomyopathy
158/270 (58.5%)
174/280 (62.1%)
0.4327
Hypertension
207/270 (76.7%)
220/280 (78.6%)
0.6100
Hyperlipidemia
204/270 (75.6%)
218/280 (77.9%)
0.5458
Coronary Artery Disease
182/270 (67.4%)
202/280 (72.1%)
0.2290
History of MI
134/270 (49.6%)
137/280 (48.9%)
0.9320
Diabetes Mellitus
130/270 (48.1%)
139/280 (49.6%)
0.7337
AFIB
120/270 (44.4%)
135/280 (48.2%)
0.3932
COPD
76/270 (28.1%)
83/280 (29.6%)
0.7078
ACE/ARB use
205/270 (75.9%)
222/280 (79.3%)
0.3584
Beta Blocker use
243/270 (90.0%)
256/280 (91.4%)
0.6595
[1]
Wilcoxon Rank-Sum Test for continuous measures and Fisher's exact test for categorical measures.
14