1-4
REM
staR
M s
ERiEs
U
sER
M
anUal
1.3.3 C
ontRaindiCations
When assessing the relative risks and benefits of using this equipment, the clinician should
understand that this device can deliver pressures up to 20 cm H
2
O. In the event of certain fault
conditions, a maximum pressure of 30 cm H
2
O is possible. Studies have shown that the following
pre-existing conditions may contraindicate the use of CPAP therapy for some patients:
• Bullous Lung Disease
• Pathologically Low Blood Pressure
• Bypassed Upper Airway
• Pneumothorax
• Pneumocephalus has been reported in a patient using nasal Continuous Positive Airway Pres
-
sure. Caution should be used when prescribing CPAP for susceptible patients such as those
with: cerebral spinal fluid (CSF) leaks, abnormalities of the cribriform plate, prior history of
head trauma, and/or pneumocephalus. (Chest 1989; 96:1425-1426)
The use of positive airway pressure therapy may be temporarily contraindicated if you exhibit
signs of a sinus or middle ear infection. Not for use with patients whose upper airways are by
-
passed. Contact your physician if you have any questions concerning your therapy.
1.4 s
ystEM
o
vERviEW
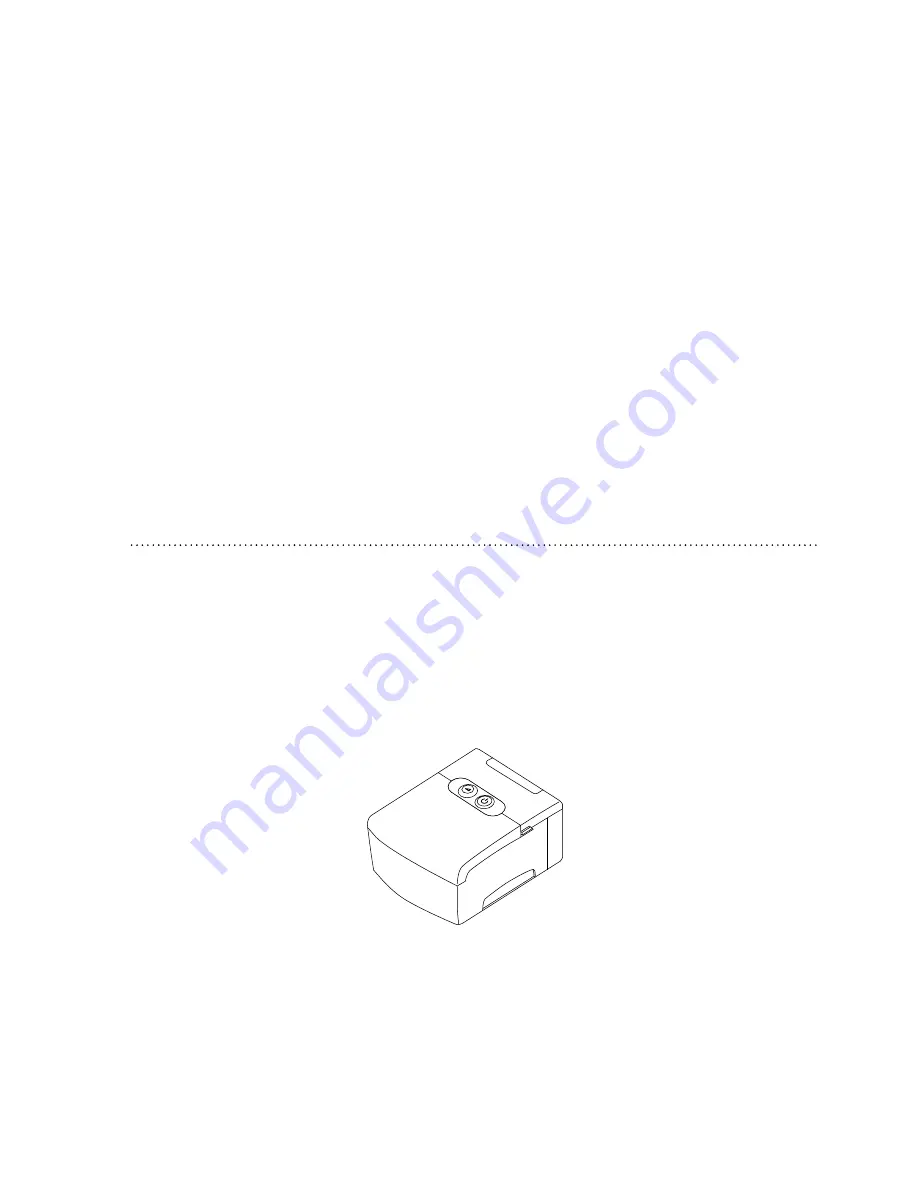
The REMstar M Series device, shown in Figure 1–2, is a sleep apnea therapy system that
delivers Continuous Positive Airway Pressure (CPAP). CPAP maintains a constant level of pres
-
sure throughout the breathing cycle.
When prescribed for you, the device provides a special feature to help make your therapy more
comfortable. The ramp function allows you to lower the pressure when you are trying to fall
asleep. The air pressure will gradually increase until your prescription pressure is reached. You also
have the option of not using the ramp feature at all.
f
igURE
1–2 REM
staR
M s
ERiEs
d
EviCE









































