EN
96200-xxx_00 GA USV Tropic 2.0_20160229.docx
- 59 -
Nebulizer chamber lid
1
(
transparent
)
cleaner
Bacteria and virus filter
Disposable items: replace after no later than 1 week or in accordance with particular
manufacturer instructions. If a closed sterile water system is used, it is recommended
to replace the filter after 48 hours of operation or every 14 days.
Medication container
intended for single use
Medication container lid
Housing
wipe with moist cloth wipe disinfection
not allowed
Power cable
Accessory sets
1.
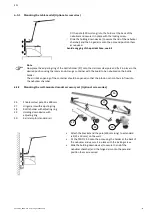
Rinse the nebulizer chamber and nebulizer chamber lid with distilled water after every use. Disinfection must
be performed with common medical disinfectants.
The quartz module remains tightly screwed into the
nebulizer chamber.
2.
After the specified contact time and with the solution diluted (according to manufacturer's specifications), the
components must be cleaned from residual disinfectant with a moist cloth and then dried.
3.
Caution, hot steam accelerates the natural aging of plastics. The material change can affect the functionality
of the device parts.
Examples of surface disinfectant:
-
Incidin® Plus *
-
Incidin® Perfect *
-
Antiseptica Combi Surface *
-
Antifect® FF *
-
B 10 *
Examples of device disinfectants:
-
Lysetol® AF *
-
Gigasept® FF *
-
Sekusept® forte S*
* registered trademarks of the respective manufacturers
All individual parts must be rinsed with distilled water after every use.
Disposable items must not be reprocessed due to risk of infection. The above-mentioned examples for disinfectants
are suggested by the device manufacturer. The recommendation of the listed active ingredient basis (pH 4.5-8)
applies. This does not release the user from consulting the hygiene expert in individual cases or checking the
components to be disinfected for compatibility with the active ingredients.
9.4
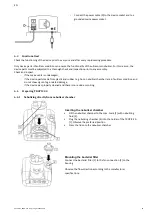
Manual cleaning of the quartz
For cleaning, remove the nebulizer chamber lid (18) from the nebulizer chamber (17) and screw the quartz module
(20) out of the nebulizer chamber (17). Reassembly is performed in reverse order.
9.5
Cleaning the automatic feeder set
Information can be found in the separate instructions for use
10
Preventive maintenance / repair
10.1
General
Caution, risk to health!
The ultrasonic nebulizer is used for treating patients. The device or parts thereof can be contaminated.
This is why, prior to returning it for inspection or repair, the nebulizer chamber, the automatic feeder set,
the bacterial filter and all hoses must be removed and the device must be cleaned and disinfected.
10.2
Preventive maintenance
The ultrasonic nebulizer is maintenance-free. The operating personnel is not required to perform preventive
maintenance other than normal care. To ensure functionality of the product, visual and functional tests must be
performed prior to use. In order the ensure operational safety of the product and availability of all functions as well as
increased life span, we recommend yearly preventive maintenance performed by an authorized specialist