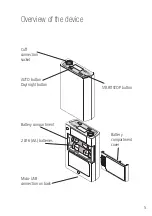
8
Introduction
Dear customer, thank you for purchasing this boso blood pressure
measuring device. The boso brand offers optimum quality and accuracy.
According to an survey among doctors conducted by the German
market research company GfK and published in January 2016, 96%
of all German general practitioners and internal medicine specialists
currently use boso blood pressure meters. This device has undergone
our strict quality control procedures and will be a reliable partner in
monitoring your patients’ blood pressure.
Please read this manual thoroughly before using the device for the
first time, as correct blood pressure readings can only be obtained
if the device is used correctly.
In this manual, the
symbol indicates action to be taken by the user.
Please contact your specialist supplier or the manufacturer for help in
preparing for first use, using the device or maintenance (contact details
are given on the inside back cover of this manual).
If you sell the device, give the buyer this manual.
This blood pressure measuring device complies with the European
requirements on which the German Medicinal Products Act is based
(ref.: CE 0124) as well as the international standard IEC 80601-2-30:
“Particular requirements for basic safety and essential performance of
automated non-invasive sphygmomanometers”.
In accordance with the Medicinal Products Operator Decree, metrology
tests (see page 29) must be conducted at regular intervals when the
device is used in a medical context.
Intended use
Non-invasive recording of systolic and diastolic blood pressure and
pulse of people over a period of 24 hours in most cases.