10 Understanding the „Lourdes“
(+)
1 x O
2
(-)
2 x H
2
(+)
1 x O
2
(-)
2 x H
2
(+)
1 x O
2
(-)
2 x H
2
Electrolysis in a
water ionizer
Electrolysis in the
Lourdes
Elektrolysis in the
Booster / HIM
!
!
!
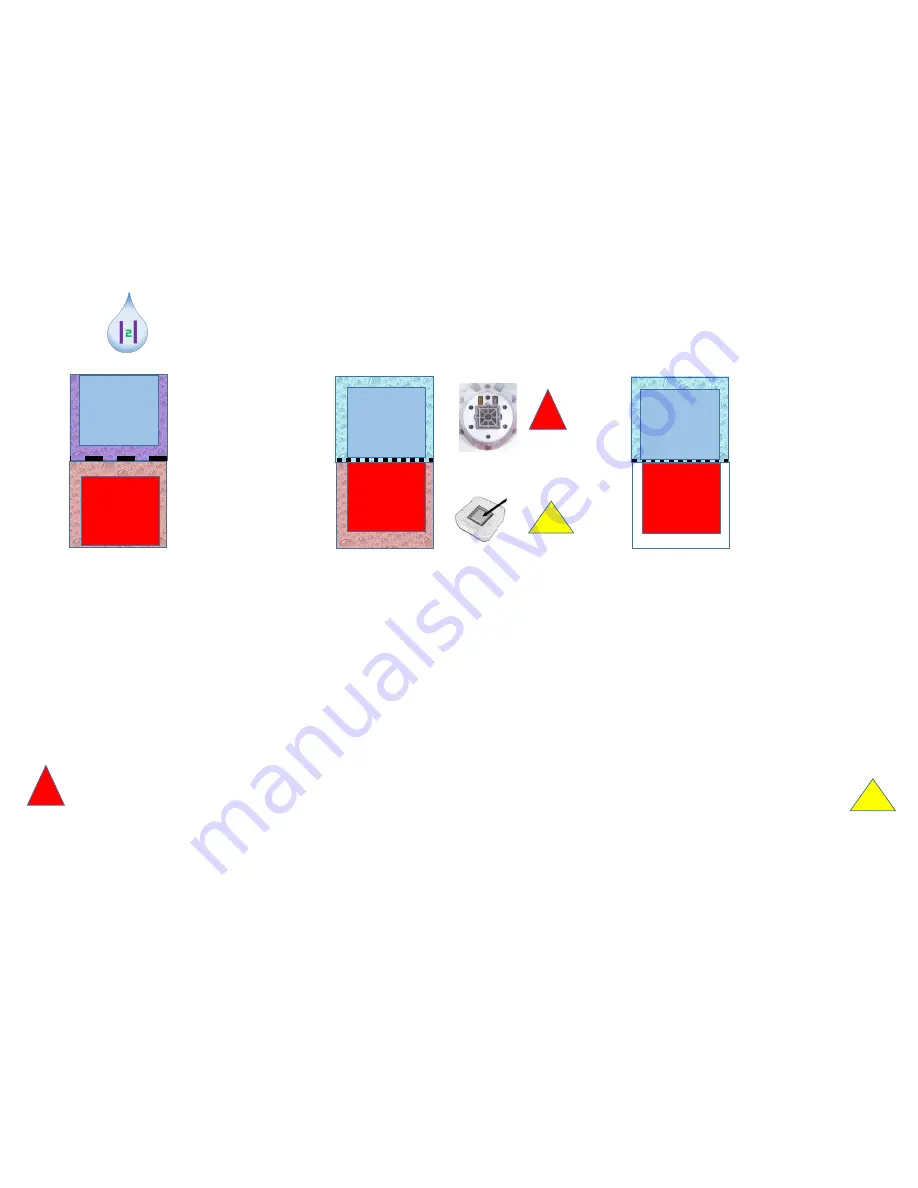
The graph above shows the differences from other electrolytic hydrogen generators. Hydrogen is also
produced in water ionizers, hydrogen boosters, or Hydrogen Infusion Machines (HIMs) through the use of
direct current to electrolyze (split) water molecules (H2O). At the end of the electrolysis process, the
divided water molecules produce the two gases hydrogen (H2) and oxygen (O2) separated by a
membrane in a ratio of 1: 2. There are different methods for this:
•
Water ionizer:
Both electrodes in water away from the membrane. The membrane has large pores. The
hydrogen water above becomes alkaline, below acidic.
•
Lourdes:
Both electrodes in the water directly on the membrane ("zero distance electrolysis" or SPE = Solid
Polymer Electrolyte). The membrane has small pores and allows only protons to go through (PEM =
proton exchange membrane). The H2-water above remains in the same pH range, below it becomes
acidic.
The sensitive membrane at the bottom of the jug should not be touched or cleaned
mechanically. An empty jug should always stand on the wet anode sponge to prevent the membrane
from drying out.
•
Booster / HIM:
Both electrodes directly on the PEM membrane, but only the upper one in the water.
Hydrogen water above remains the same in pH. Oxygen escapes into the air.
!






































