In order to allow these phases it is essential
to enrich the used gas with oxygen to make it
possible to be breathed additional times. The
enrichment is provided by a constant flow
added to exhaled gas after this one is cleaned
by the filter; to make this possible only and
exclusively breathable mixes as Heliox
(Oxygen-Helium) or Nitrox can be used,
according to the depth reached; common air
cannot absolutely be used.
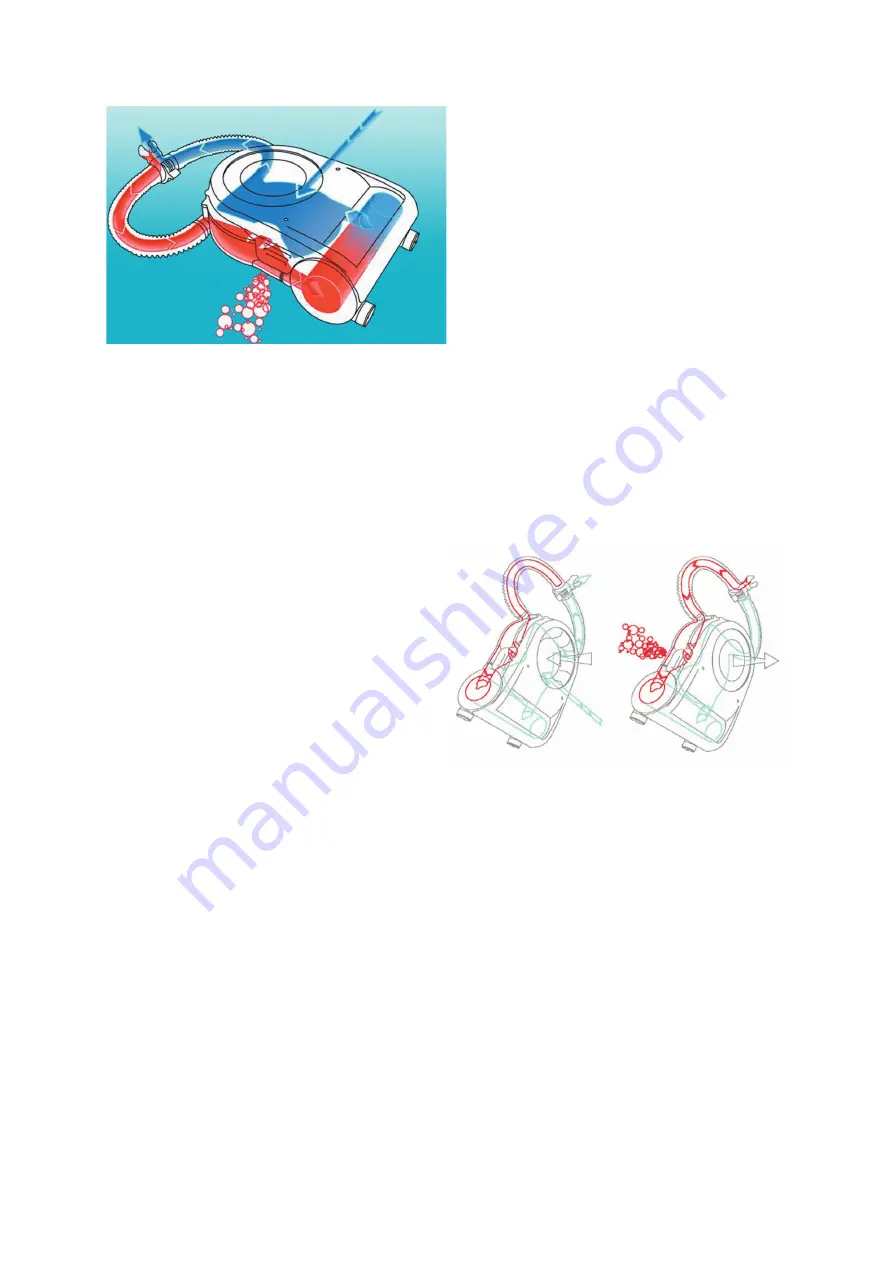
As we said, the
channeled
route of breathed
and exhaled gas is sealed; through a red
wrinkled hose it enters in a section that contains the exhaust valve linked to the counterlung
big membrane by a thin tube and it arrives to the big counterlung that lifts the exaust valve
membrane operating the opening during the phase of maximum expansion; this way the last part
of the exhaled gas –containing the maximum carbon dioxide concentration- is vented outside;
the exhaled gas staying in the loop enters the see-through container and then it goes into the
filter, where the filtering material
chemically removes
carbon dioxide. The flux of cleaned gas
goes into the counterlung and joins the continuous flow of mixture enriched with oxygen that
arrives from the tank through the injector and through the black wrinkled hose, it is breathed in
again and again for several times.
An agreeable collateral effect of these
subsequent cycles is the progressive rise in
temperature of the breathed gas that will not
make the diver notice water temperature,
even when it is cold.
Physicians claim that a diver’s oxygen
consumption can be an average of 1,5 lt. per
minute (1 lt. at rest and 2 lt. under pressure)
and that the oxygen partial pressure, resulting from the relation between the percentage of
oxygen in the breathable mixture and the ambient pressure, must be less than 1.4/ maximum1.5
bars; so the percentage of oxygen present in the breathed gas and the quantity of constant flux
(liters per minute) must be determined in advance according to the planned maximum depth
of the dive. In short, the greater the depth of the dive will be, the greater will be the ambient
pressure and consequently the smaller must be the percentage of oxygen present in the breathed
gas; in our case the considered values are as follow:
maximum depth 22 mt./ 73 ft. = 50% oxygen = flux 6/6,5 liters/minute (on request)
maximum depth 30 mt./100 ft. = 40% oxygen = flux 9/9,5 liters/minute (standard)
maximum depth 40 mt./133 ft. = 32% oxygen = flux 14/14,5 liters/minute (on request)
As an example:in the case of 30 mt./100 ft. of depth, with a
mixture
having the 40%
of
breathable
oxygen
, the partial pressure will be: 0,40
(
oxygen quantity
) x 4ATA (
ambient pressure
) = 1.;, that is the
largest and the most critical value if breathed on open circuit, correct and normal if breathed
with a rebreather as it has not to be forgotten that after a few breathings the percentage of
oxygen in the counterlung can be even reduced of about 5% and this has to be considered also
for the dive time calculation.



































