CAUTION
READ AND SAVE THESE INSTRUCTIONS
A patient safety strap is required during all procedures. Follow normal and required safety protocol for all procedures
where the patient is in an elevated position for the procedure (straps, attendants, etc.). Always be certain that attending
staff is aware of the patient’s position while the device is in use. Reposition the patient if necessary to promote stability.
Due to the increased distance between the patient and the table surface, additional safety measures are recommended
when the table top is not used in a level position due to the risk of the patient falling off the table.
The Oakworks® Spine Positioning System II is not designed for use with diagnostic x-ray systems where the x-ray
generator is located above the radiographic table and the film cassette or image intensifier is located below the radio-
graphic table. The X-Ray generator must be located below the radiographic table. The Spine Positioning System II is not
designed for use with magnetic resonance imaging systems. The Spine Positioning System II is not intended for use in
cranial procedures.
Do not overhang the radiolucent frame beyond the warning line on the frame.
Operate the C-arm of the fluoroscopy system with the Spine Positioning System II in place before using the device with
a patient for the first time. Make sure there is adequate clearance to permit free C-arm rotation for both the patient and
the positioning device.
Do not permit the patient to push down on the Crescent Face Pad in an effort to lift themselves up while dismounting the
platform and/or the table.
The Spine Positioning System II should generally not be used when a patient is under general anesthesia, especially
when prolonged cases are performed. This will reduce the risk of ocular or facial nerve injury.
The cushioning foam contained within the Torso Support will lose its ability to spring back to the original position over
time and the amount of foam compression will increase. Therefore, the Torso Support should be replaced periodically to
ensure the device functions as intended.
To prevent the potential of cross-contamination, it is strongly advised to use barrier techniques when the device is
in use. A disposable or laundered patient gown, or disposable pad are satisfactory for use as a barrier for the Torso
Support and other components and accessories, except when the patient presents with pathology that would indicate
otherwise. A disposable face rest cover should be used to cover the Face Rest Pad. Contact Oakworks for ordering
information. Barrier techniques should be used in addition to disinfection procedures, not in lieu of them.
Be sure to support the weight of the patient’s head while making adjustments to the cervical positioning feature of the
Platform Frame. Make sure all cam locks are secure before relinquishing support of the positioning assembly.
DANGER
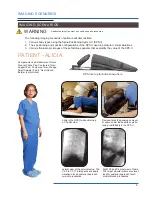
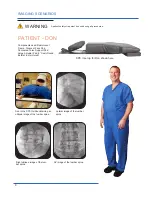
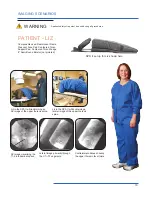
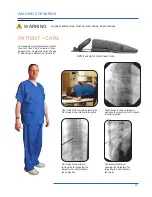
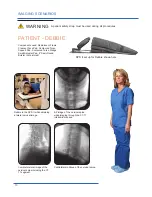
The Cervical Support System has metal parts that can cause back scatter of x-rays, see Product Description for photo.
When x-rays are present, wear a suitable radiation barrier.
The Spine Positioning System II is constructed using metal pins in the Quick Cam Locks and aluminum tubing in the
support structure. These are out of the field of view in most A-P and oblique tilted views. Place the positioning assembly
according to the recommendations in the directions for use to eliminate, or reduce any artifacts. If artifacts still remain
to the extent that they would compromise the efficacy of needle placement, discontinue use of the device during the
affected procedure.
The Spine Positioning System II is designed to be a standalone product used with radiographic equipment. It must not
be modified or incorporated into any other equipment.
All materials used in the construction of the device and accessories are safe for temporary and moderately frequent
human contact. The device is not intended for prolonged contact.
Do not use the Face Rest Support Arms as a handle to carry the Spine Positioning System II.
Follow maintenance instructions found near the end of this manual. Mechanical components should be checked periodi-
cally to insure that they are functioning properly to insure the safety of the patient.
SPS II weight limit: 350 lbs. (159 kg.) Crescent Face Pad Support weight limit: 25 lbs. (11 kg.)
IMPORTANT SAFETY INSTRUCTIONS
2
IMPORTANT SAFETY INSTRUCTIONS
Содержание Spine Positioning System II
Страница 22: ...THIS PAGE IS INTENTIONALLY LEFT BLANK 19...
Страница 23: ...20 THIS PAGE IS INTENTIONALLY LEFT BLANK...