Dipolar nature of water
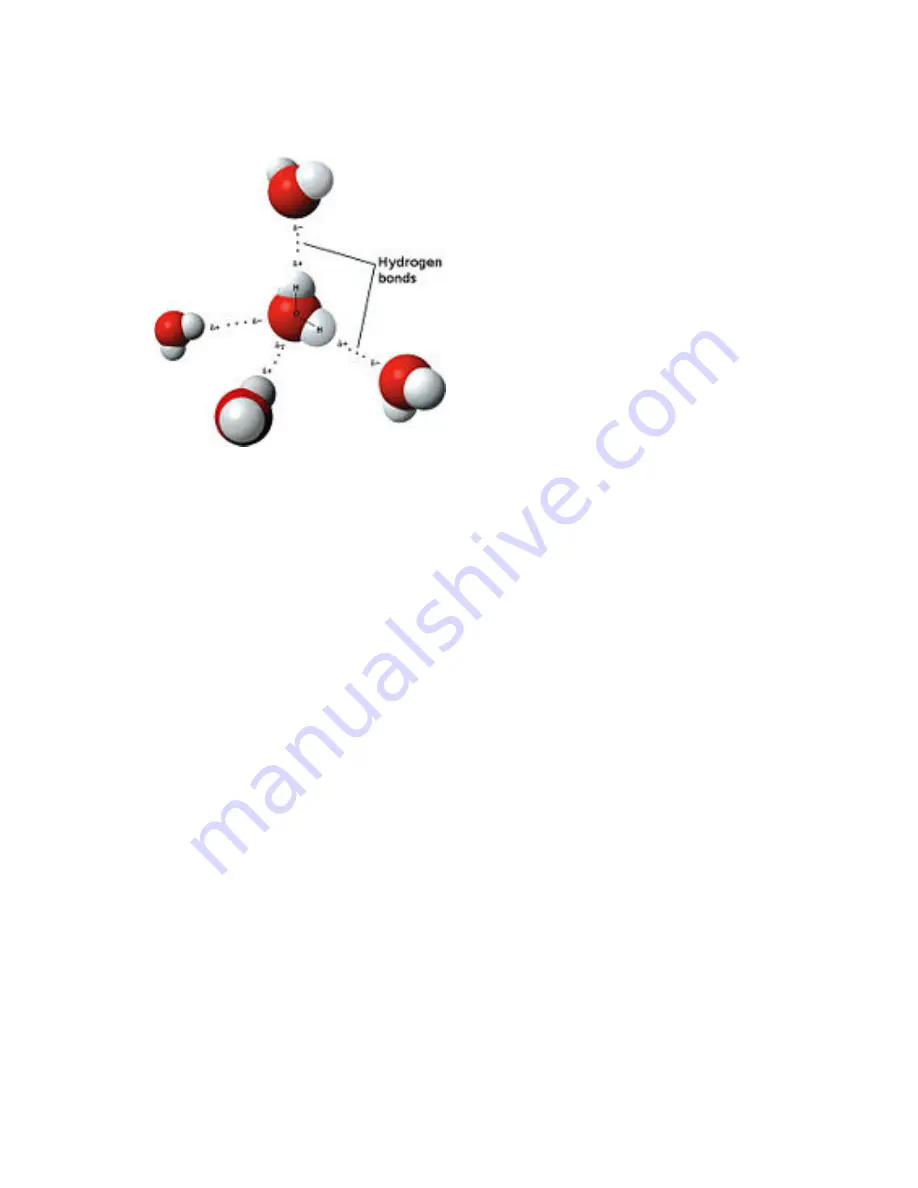
Model of hydrogen bonds between molecules of water
An important feature of water is
its polar nature. The water molecule
forms an angle, with hydrogen atoms at
the tips and oxygen at the vertex. Since
oxygen has a higher electro-negativity
than hydrogen, the side of the molecule
with the oxygen atom has a partial
negative charge. A molecule with such a
charge difference is called a dipole. The
charge
differences
cause
water
molecules to be attracted to each other
(the relatively positive areas being
attracted to the relatively negative
areas) and to other polar molecules.
This attraction is known as hydrogen
bonding, and explains many of the properties of water. Certain molecules, such
as carbon dioxide, also have a difference in electro-negativity between the atoms
but the difference is that the shape of carbon dioxide is symmetrically aligned
and so the opposing charges cancel one another out. This phenomenon of water
can be seen if you hold an electrical source near a thin stream of water falling
vertically, causing the stream to bend towards the electrical source.
Although hydrogen bonding is a relatively weak attraction compared to the
covalent bonds within the water molecule itself, it is responsible for a number of
water's physical properties. One such property is its relatively high melting and
boiling point temperatures; more heat energy is required to break the hydrogen
bonds between molecules. The similar compound hydrogen sulfide (H
2
S), which
has much weaker hydrogen bonding, is a gas at room temperature even though
it has twice the molecular mass of water. The extra bonding between water
molecules also gives liquid water a large specific heat capacity. This high heat
capacity makes water a good heat storage medium.
Hydrogen bonding also gives water its unusual behavior when freezing.
When cooled to near freezing point, the presence of hydrogen bonds means that
the molecules, as they rearrange to minimize their energy, form the hexagonal
crystal structure of ice that is actually of lower density: hence the solid form, ice,
will float in water. In other words, water expands as it freezes, whereas almost all
other materials shrink on solidification.
An interesting consequence of the solid having a lower density than the
liquid is that ice will melt if sufficient pressure is applied. With increasing pressure
the melting point temperature drops and when the melting point temperature is
lower than the ambient temperature the ice begins to melt. A significant increase
of pressure is required to lower the melting point temperature —the pressure
exerted by an ice skater on the ice would only reduce the melting point by
approximately 0.09 °C (0.16 °F).
Содержание WaterCrest
Страница 1: ...Owner s Manual 2015...
Страница 7: ...evaporation transpiration condensation precipitation and runoff...
Страница 13: ......
Страница 14: ......
Страница 16: ...Installation Procedure...
Страница 21: ...Contact Information a division of Waukesha Wisconsin USA www hanishwater com info hanishwater com...





































