Reviews:
No comments
Related manuals for SURGmatic S11 L
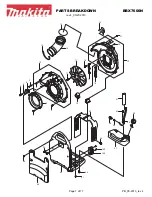
BBX7600N
Brand: Makita Pages: 17

BBX7600
Brand: Makita Pages: 13

HS-2200
Brand: Datavideo Pages: 48

HDR-70
Brand: Datavideo Pages: 34

HDR-70
Brand: Datavideo Pages: 3

DN-600
Brand: Datavideo Pages: 34

TVS-1000
Brand: Datavideo Pages: 8

MS-900
Brand: Datavideo Pages: 10

Tablet1000 Series
Brand: Hantek Pages: 98

Logic
Brand: Olsen Pages: 58

ME-9430
Brand: PASCO Pages: 30

APT-9411 Series
Brand: TAKAYA Pages: 77

TR-800XXL4
Brand: Tadano Pages: 402

HQ Series
Brand: Hach Pages: 456

MB2
Brand: B-K lighting Pages: 2

MRB 3000 SDD
Brand: B medical systems Pages: 28

Pro hunter 2525
Brand: SprotDog Pages: 28

MK8 90
Brand: Barikell Pages: 32

















