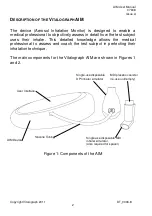
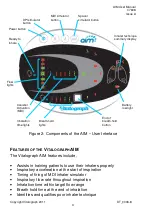
AIM User Manual
07608
Issue A
Copyright Vitalograph 2011 DT_0006-8
8
C
LEANING
I
NSTRUCTIONS
Cleaning and Disinfecting the Vitalograph AIM
A new disposable inhaler simulator should be used for each subject.
The frequency of cleaning and disinfecting is dependent on the
Facility’s Risk Assessment, usage, and test environment.
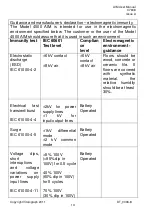
Table of Materials Used & Cleaning/Disinfection Methods
This listing of materials used is given to provide users with information to allow the assessment of
other cleaning and disinfecting procedures available in the facility on this device.
Part
Material Clean/
Disinfect
Autoclave Recommended
Disinfectants
Top
Case
Exterior
ABS
Clean
No
Bottom
Case
Exterior
AMS
Clean
No
Overlay
label
PET Film
Clean
No
White
Silicone
Tubing
Silicone
Clean
Viable
Wiping with a 70% isopropyl alcohol
impregnated cloth provides a suitable
form
of
cleaning
and
low-level
disinfection. This may be preceded by
cleaning with an anti-static foam
cleaner if necessary.
Note: Always follow the safety guidelines given by the manufacturer of
cleaning and disinfectant chemicals.
All external parts of the Vitalograph AIM require
cleaning,
i.e. the
removal of visible particulate contamination. The AIM is not designed
as a ‘sterile’ device.
Definitions of cleaning and disinfection are as defined in “Sterilization,
Disinfection and Cleaning of Medical Equipment: Guidance on
Decontamination from the Microbiology Committee to Department of
Health Medical Devices Directorate, 1996”.
Recommendations for chemical disinfectants are derived from the
PHLS publication “Chemical Disinfection In Hospitals 1993”.