EN
5
Ozone generator Airozon® 28 ECO
Information about the device
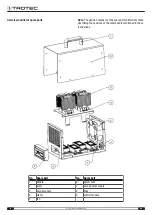
Device description
Ozone is a very powerful oxidising agent with the ability to
eliminate bacteria, viruses, gases and toxins. The oxidising
agent ozone breaks down the existing molecules. The ozone is
generated by the device in an electrical discharge procedure
and emitted to the room air at a high concentration. You can use
the device to eliminate e.g. pathogens, but also cooking smells
or musty odours as well as burnt smell. The applied method is
similar to the natural air purification during a thunderstorm.
Operating principle
The ozone is generated through high-voltage discharge. The
high voltage creates extremely high electric field strengths in
the discharge unit. This leads to numerous brief barrier
discharges between the electrodes.
The integrated fan sucks in ambient air through the air filter at
the rear of the device, leads it past the ozone unit and then
emits the air that now contains ozone back into the room.
No chemicals are required for the ozone generation, hence this
process causes no damage to the environment. When the
device has been switched off and the cleaning process has
been completed, the remaining ozone in the room air will
decompose into normal divalent oxygen (O
2
).
Formation, perception and distribution of ozone
The word ozone has become an integral part of our vocabulary
and the colourless, toxic gas is part of our everyday life. The
irritant effect ozone can have on our eyes and airways has been
common knowledge for a few years now, especially since the
increasing ozone volume can be attributed to the so-called
summer smog. Ozone is further generated during certain
industrial processes.
Ozone (chemical symbol: O
3
) consists of three oxygen atoms.
Ozone is created wherever oxygen molecules (O
2
) in the air are
turned into atoms (O) due to electrical energy or UV radiation. It
is these atoms that can react with the oxygen molecules to form
ozone (O
3
).
In case of a high solar irradiation ozone forms involving other air
pollutants. The nitrogen oxides (NOx) from automobiles,
domestic heating systems, power plants and the industry play a
major role in this.
Depending on the concentration, ozone can have a very intense
smell similar to chlorine, hay or carnations, odours that can be
smelled in mountain areas. By nature, the human nose is
already vastly superior to most measuring devices: It detects the
gas with a concentration of as little as 0.01 ml/m
3
. What this
means when compared to other irritant gases is that we
humans can already detect even minor quantities of ozone
which gives us the opportunity to take corresponding
precautions to avoid any hazard caused by the gas in due time.
However, our nose also has a crucial disadvantage compared
with measuring devices: the so-called
habituation effect
. After
only a brief period of time spent in an ozone-polluted
environment we become so acclimated to the smell that we go
noseblind.
The impact of ozone on human beings
The sensitivity to ozone depends on its concentration and varies
for each individual. Ozone is an oxidative irritant gas, affecting
eyes, nose, pharynx and lungs even at low concentrations. The
mucous membranes are unable to stop it seeing as ozone is
hardly soluble in water. As a result, the gas can be carried
deeper into the lungs than other irritant gases. Concentrations
of 200 µg/mm
3
and higher can cause the following symptoms:
•
irritations of the mucous membranes, eyes and the
respiratory tract
•
hoarseness, coughs and headaches
•
feeling of constriction behind the sternum
•
reduced physical performance
The main damage is caused in the respiratory tract which can
lead to breathing difficulties and a reduced respiratory volume.
Late complications can include nosebleeds, a bronchitis (or
tracheitis) or a pulmonary oedema. But the transition from
irritations without lasting consequences to long-term changes
with pathological significance is very smooth.