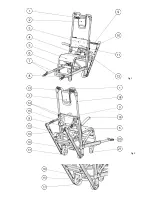
8
Legislative Decree 25/01/2010, n. 35
Modifications and additions to the 20/02/97 Decree n. 46
UNI EN ISO 14971
Application of risks managing to medical devices
UNI CEI EN ISO 15223-1
Medical devices - Symbols for use in the medical device labels,
labelling and information to be provided. Part 1: general requirements
UNI CEI EN 1041
Information supplied by the medical devices manufacturer
CEI EN 62366
Medical Devices - Application of the utilisation characteristics of
engineering to medical devices
MEDDEV 2.4/1a-b
Guideline for the classification of medical devices
NB-MED 2.5.1/Rec 5
Technical Documentation
MEDDEV 2.7.1
Clinical Data
MEDDEV 2.12/1
Medical Devices vigilance system
UNI EN 14155
Clinical evaluation of the medical devices for human beings - Part 2:
Clinical evaluation plans
3.6
Environmental conditions
Functioning temperature:
from -15 to +50 °C
Storage temperature:
from -20 to +60 °C
4
OPERATING INSTRUCTIONS
4.1
Transport and storage
Before transporting the appliance, make sure that it is correctly packaged ensuring also that there are no risks of shocks,
bumps or falls during the transport itself. Keep the original packaging for use in case of any further transport and for storage.
Damage to the appliance caused during transport and handling is not covered by the guarantee. Repairs or replacement of
the damaged parts are the responsibility of the client. The device must be stored in a dry, cool area away from direct
sunlight. It must not be placed in contact with any substances or chemical agents which could cause damage and reduce
safety characteristics.
During storage, do not placed heavy materials over the device. The chair should not be considered and used as a shelf for any
type of material.
4.2
Preparation
On receipt of the product:
Remove the packaging and display the material so that all components are visible.
Check that all the components/pieces on the accompanying list are present.
The appliance must be checked before every use so as to reveal any working abnormalities and/or damage caused by
transport and/or storage. In particular, check:
General functionality of the device
Cleanliness of the device (remember that the failure of cleaning may cause the risk of cross infections)
Absence of cuts, holes, tears on the structure, including the straps
Correct fixation of all nuts, bolts and screws
Correct fixation of straps
Correct fastening of straps
State of use (moving parts, wheels, belts)
Integrity of sewings and sheets
No piping or metal sheet present bends or cracks
The seat and the backrest do not present any lacerations and cuts
Weldings are intact, without any cracks or breaks
The seat belts, sheets, moving parts, wheels and handles are intact and functioning
The wheels are fastened securely, are stable and running
The wheels are free of debris
The front-wheels are self-positioning (if equipped)
The brake works correctly and is operated simultaneously
The device opens and locks
The device closes automatically
The slides open
The slides are automatically closed (if equipped)
The belts of the sleds run and have always the correct tension for being used
Functioning of springs
The control system ESC can be selected (if equipped)
Summary of Contents for EVA
Page 6: ...6 Fig 1 Fig 2 ...