UK
alkaline earth salts. Owing to the effects of CO2, a large proportion of these salts is represented
by carbonates. By de
fi
nition, the proportion of calcium and magnesium salts represented as
carbonate is described as carbonate hardness.
As a rule, the carbonate hardness is below the total hardness value. In certain exceptional cases
(e.g. many tropical waters) the carbonate hardness can be higher than the total hardness.
Most freshwater
fi
sh and plants in an aquarium thrive well at a carbonate hardness of roughly
between 3 and 15°d. For successful CO2 fertilization, the carbonate hardness should not fall
below 4 to 5°d. For optimized pH buffering action in saltwater, a carbonate hardness around
7-10°d ought to be maintained.
In the garden pond the carbonate hardness plays a vital role as a stabiliser for the pH level.
Green
fl
oating algae (green water) in particular “consume” carbonate hardness by rapid
assimilation, driving the pH up to levels which are dangerous for
fi
sh (above 9). Carbonate
hardness levels of at least 5° d should therefore be maintained in garden ponds.
What to do in the event of unfavourable values
There are various methods for reducing water hardness (e.g. by using a reverse osmosis unit
such as the JBL Osmose 120). Ask your pet shop specialist for details. Use JBL Aquakal or
JBL AquaDur plus to increase the carbonate hardness in freshwater aquariums. Use JBL
CalciuMarin in marine aquariums.
In garden ponds the carbonate hardness can be increased by the addition of JBL Alkalon combi.
Instructions
1. Repeatedly rinse the test cup with the water to be tested.
2. Fill the test cup with the water to be tested up to the 5 ml mark (CAUTION: the lower line of
the water level must coincide with the marking).
3. Add reagent one drop at a time, counting the drops, and agitate after each drop until the
colour changes from blue to yellow-orange.
4. One drop of reagent solution used corresponds to 1° carbonate hardness (German scale).
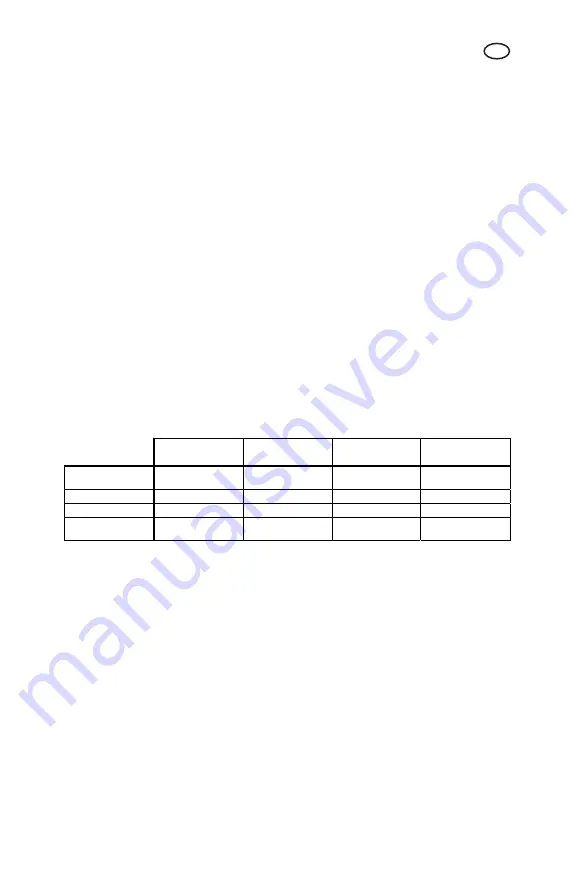
See table below for conversion into other commonly accepted units of measurement.
For further details on the signi
fi
cance of hardness in the biological system of your aquarium,
see the JBL brochure entitled „Was - Wie - Warum?“ (What - How - Why?), No. 2 or JBL
brochure No. 8 for garden ponds.
Our tip for ecologically-minded users
All reagents for the JBL Test Sets are also available from your retailer as reasonably priced re
fi
lls.
pH 3.0 - 10:
Features
The JBL pH Test Set 3.0-10 is an easy-to-use, quick test for general guidance and control of the
pH level in freshwater and saltwater, covering the wide pH-range 3.0 to 10.
Why test the pH level?
The well-being of
fi
sh, invertebrates and the growth of aquatic plants are largely dependent
on constantly maintaining a suitable pH level. Many substances dissolved in water are also
liable to changes caused by the pH level. Fluctuations in the pH level, in particular, ought to
be avoided.
The pH level most conducive for keeping the majority of freshwater
fi
sh and plants is in
Carbonate
hardness
Acid capacity
mmol/l
German degree °d
French degree °f
Hydrogen
carbonate mg/l
Acid capacity
mmol/l
- 2.78
4.94
61.0
German degree °d
0.36 - 1.78
21.8
French degree °f
0.20 0.56 - 12.3
Hydrogen
carbonate mg/l
0.016 0.046 0.08
-
























