16
•
Powered muscle stimulators should not
be used by patients with demand type
cardiac pacemakers.
•
Powered muscle stimulators should not
be used during pregnancy.
•
Powered muscle stimulators should not
be used until 12 weeks after childbirth.
•
Do not use this unit while operating
machinery or while driving.
•
Stimulation should not be applied over
the carotid nerves, the neck or mouth,
transthoracically or transcerebrally.
•
Stimulation should not be applied over
swollen, infected or inflamed areas of
skin or over or in proximity to cancerous
lesions.
•
Caution should be used for patients with
suspected or diagnosed heart problems,
or epilepsy.
•
Caution should be used where there is a
tendency to haemorrhage following acute
trauma or fracture, following recent
surgical procedures when muscle
contraction could disrupt the healing
process, over the menstruating uterus,
over areas of skin which lack normal
sensation.
•
Some patients may experience mild skin
irritation due at the site of the electrodes.
This can sometimes be reduced by using
alternative electrodes and/or
repositioning them.
•
Electrode placement and stimulation
settings should be based on the guidance
of the prescribing practitioner.
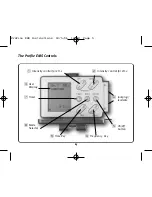
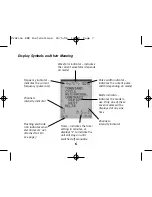
Contraindications and Precautions
Profile EMS Instructions 18/5/06 10:19 Page 17