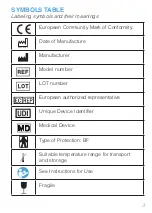
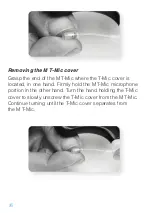
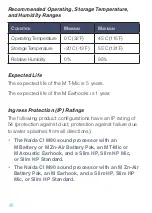
5
PURPOSE AND SCOPE OF IFU
This IFU is designed to help cochlear implant recipients,
and their caregivers if applicable. to understand the use
and care of the M T-Mic and M Earhooks. It is also meant to
be used by hearing care professionals trained in the fitting
of cochlear implants to counsel recipients on the use of
the M T-Mic and M Earhooks and to troubleshoot problems.
Limitations and Contraindications
As the M T-Mic and M Earhooks are a component of
the HiResolution
TM
Bionic Ear System, the following
contraindications stated for the HiResolution Bionic Ear
System are applicable: Deafness due to lesions of
the acoustic nerve or central auditory pathway; cochlear
ossification that prevents electrode insertion; absence of
cochlear development.
Precautions, Cautions, and Warnings
•
This device should be used only by the individual for
whom it is prescribed.
•
CHOKING HAZARD: Contains small parts that pose
a hazard of inhalation, choking, or ingestion. If any
parts are swallowed or inhaled, consult a physician or
hospital immediately.
•
Do not leave children unattended with or allow children
to play with the M T-Mic or M Earhook.
•
Do not allow children to place the M T-Mic or
M Earhook in the mouth.