1
TO ENSURE PROPER DEPLOYMENT AND USE OF THIS DEVICE AND TO PREVENT INJURY TO PATIENTS, READ ALL INFORMATION CONTAINED IN THESE INSTRUCTIONS
FOR USE.
CAUTION: Federal law restricts this device to sale by or on the order of a physician.
The instructions for use is recyclable. Dispose of used product and packaging following standard solid biohazard waste procedures.
DEVICE DESCRIPTION
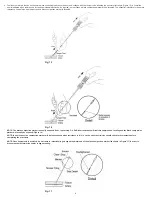
7KH$QJLR6HDO9,39DVFXODU&ORVXUH'HYLFHFRQVLVWVRIWKH$QJLR6HDO9,3GHYLFHDQLQVHUWLRQVKHDWKDQDUWHULRWRP\ORFDWRUPRGL¿HGGLODWRUDQGDJXLGHZLUH7KH$QJLR6HDO9,3
GHYLFHLVFRPSRVHGRIDQDEVRUEDEOHFROODJHQVSRQJHDQGDVSHFLDOO\GHVLJQHGDEVRUEDEOHSRO\PHUDQFKRUWKDWDUHFRQQHFWHGE\DQDEVRUEDEOHVHOIWLJKWHQLQJVXWXUH6767KHGHYLFH
VHDOVDQGVDQGZLFKHVWKHDUWHULRWRP\EHWZHHQLWVWZRSULPDU\PHPEHUVWKHDQFKRUDQGFROODJHHPRVWDVLVLVDFKLHYHGSULPDULO\E\WKHPHFKDQLFDOPHDQVRIWKHDQFKRU
DUWHULRWRP\FROODJHQVDQGZLFKZKLFKLVVXSSOHPHQWHGE\WKHFRDJXODWLRQLQGXFLQJSURSHUWLHVRIWKHFROODJHQ7KHGHYLFHLVFRQWDLQHGLQDGHOLYHU\V\VWHPWKDWVWRUHVDQGWKHQGHOLYHUV
WKHDEVRUEDEOHFRPSRQHQWVWRWKHDUWHULDOSXQFWXUH7KHGHOLYHU\V\VWHPIHDWXUHVD6HFXUH&DSWKDWIDFLOLWDWHVSURSHUWHFKQLTXHIRUGHOLYHU\DQGGHSOR\PHQWRIWKHDEVRUEDEOHXQLW7KH
$QJLR6HDO9DVFXODU&ORVXUH'HYLFHFRPSRQHQWVDUHQRWPDGHIURPODWH[UXEEHU7KLVSURGXFWLV05VDIH
INDICATIONS
7KH$QJLR6HDOGHYLFHLVLQGLFDWHGIRUXVHLQFORVLQJDQGUHGXFLQJWLPHWRKHPRVWDVLVDWWKHIHPRUDODUWHULDOSXQFWXUHVLWHLQSDWLHQWVZKRKDYHXQGHUJRQHGLDJQRVWLFDQJLRJUDSK\
procedures or interventional procedures using an 8 French or smaller procedural sheath for the 8F Angio-Seal device and a 6 French or smaller procedural sheath for the 6F Angio-Seal
GHYLFH
7KH$QJLR6HDOGHYLFHLVDOVRLQGLFDWHGIRUXVHWRDOORZSDWLHQWVZKRKDYHXQGHUJRQHGLDJQRVWLFDQJLRJUDSK\WRDPEXODWHVDIHO\DVVRRQDVSRVVLEOHDIWHUVKHDWKUHPRYDODQGGHYLFH
SODFHPHQW
7KH$QJLR6HDOGHYLFHLVDOVRLQGLFDWHGIRUXVHWRDOORZSDWLHQWVZKRKDYHXQGHUJRQHDQLQWHUYHQWLRQDOSURFHGXUHWRDPEXODWHVDIHO\DIWHUVKHDWKUHPRYDODQGGHYLFHSODFHPHQW
CONTRAINDICATIONS
7KHUHDUHQRFRQWUDLQGLFDWLRQVWRWKHXVHRIWKLVGHYLFH$WWHQWLRQLVGUDZQWRWKHZDUQLQJVDQGSUHFDXWLRQV
WARNINGS
'RQRWXVHLIWKHWHPSHUDWXUHLQGLFDWRUGRWRQSDFNDJHKDVFKDQJHGIURPOLJKWJUD\WRGDUNJUD\RUEODFN
'RQRWXVHLIWKHSDFNDJHLVGDPDJHGRUDQ\SRUWLRQRIWKHSDFNDJHKDVEHHQSUHYLRXVO\RSHQHG
'RQRWXVHLIWKHLWHPVLQWKHNLWDSSHDUGDPDJHGRUGHIHFWLYHLQDQ\ZD\
'RQRWXVHWKH$QJLR6HDOGHYLFHZKHUHEDFWHULDOFRQWDPLQDWLRQRIWKHSURFHGXUHVKHDWKRUVXUURXQGLQJWLVVXHVPD\KDYHRFFXUUHGDVWKLVPD\UHVXOWLQDQLQIHFWLRQ
• Do not use the Angio-Seal device if the procedure sheath has been placed through the superficial femoral artery and into the profunda femoris as this may result in collagen
GHSRVLWLRQLQWRWKHVXSHUILFLDOIHPRUDODUWHU\7KLVPD\UHGXFHEORRGIORZWKURXJKWKHYHVVHOOHDGLQJWRV\PSWRPVRIGLVWDODUWHULDOLQVXIILFLHQF\
'RQRWXVHWKH$QJLR6HDOGHYLFHLIWKHSXQFWXUHVLWHLVDWRUGLVWDOWRWKHELIXUFDWLRQRIWKHVXSHUILFLDOIHPRUDODQGSURIXQGDIHPRULVDUWHU\DVWKLVPD\UHVXOWLQWKHDQFKRU
FDWFKLQJRQWKHELIXUFDWLRQRUEHLQJSRVLWLRQHGLQFRUUHFWO\DQGRUFROODJHQGHSRVLWLRQLQWRWKHYHVVHO7KHVHHYHQWVPD\UHGXFHEORRGIORZWKURXJKWKHYHVVHOOHDGLQJWRV\PSWRPV
RIGLVWDODUWHULDOLQVXIILFLHQF\
'RQRWXVHWKH$QJLR6HDOGHYLFHLIWKHSXQFWXUHVLWHLVSUR[LPDOWRWKHLQJXLQDOOLJDPHQWDVWKLVPD\UHVXOWLQDUHWURSHULWRQHDOKHPDWRPD
PRECAUTIONS
Special Patient Populations
7KHVDIHW\DQGHႇHFWLYHQHVVRIWKH$QJLR6HDOGHYLFHKDVQRWEHHQHVWDEOLVKHGLQWKHIROORZLQJSDWLHQWSRSXODWLRQV
3DWLHQWVXQGHUJRLQJDQLQWHUYHQWLRQDOSURFHGXUHZKRPDUHEHLQJWUHDWHGZLWKZDUIDULQ
3DWLHQWVZKRKDYHNQRZQDOOHUJLHVWREHHISURGXFWVFROODJHQDQGRUFROODJHQSURGXFWVRUSRO\JO\FROLFRUSRO\ODFWLFDFLGSRO\PHUV
3DWLHQWVZLWKSUHH[LVWLQJDXWRLPPXQHGLVHDVH
3DWLHQWVXQGHUJRLQJWKHUDSHXWLFWKURPERO\VLV
3DWLHQWVSXQFWXUHGWKURXJKDYDVFXODUJUDIW
3DWLHQWVZLWKXQFRQWUROOHGK\SHUWHQVLRQ!PP+JV\VWROLF
3DWLHQWVZLWKDEOHHGLQJGLVRUGHULQFOXGLQJWKURPERF\WRSHQLDSODWHOHWFRXQWWKURPEDVWKHQLDYRQ:LOOH%UDQG¶VGLVHDVHRFW
3HGLDWULFSDWLHQWVRURWKHUVZLWKVPDOOIHPRUDODUWHU\VL]HPPLQGLDPHWHU6PDOOIHPRUDODUWHU\VL]HPD\SUHYHQWWKH$QJLR6HDODQFKRUIURPGHSOR\LQJSURSHUO\LQWKHVH
SDWLHQWV
3DWLHQWVZKRDUHSUHJQDQWRUODFWDWLQJ
Procedure
7KH$QJLR6HDOGHYLFHLVWREHXVHGRQO\E\DOLFHQVHGSK\VLFLDQRURWKHUKHDOWKFDUHSURIHVVLRQDODXWKRUL]HGE\RUXQGHUWKHGLUHFWLRQRIVXFKSK\VLFLDQSRVVHVVLQJDGHTXDWH
LQVWUXFWLRQLQWKHXVHRIWKHGHYLFHHJSDUWLFLSDWLRQLQDQ$QJLR6HDOSK\VLFLDQLQVWUXFWLRQSURJUDPRUHTXLYDOHQW
8VHDVLQJOHZDOOSXQFWXUHWHFKQLTXH'RQRWSXQFWXUHWKHSRVWHULRUZDOORIWKHDUWHU\
,IDSDWLHQWKDVKDGDSURFHGXUHVKHDWKOHIWLQSODFHIRUORQJHUWKDQKRXUVFRQVLGHUDWLRQVKRXOGEHJLYHQWRWKHXVHRISURSK\ODFWLFDQWLELRWLFVEHIRUHLQVHUWLRQRIWKH$QJLR6HDOGHYLFH
7KH$QJLR6HDOGHYLFHVKRXOGEHXVHGZLWKLQRQHKRXURIRSHQLQJWKHIRLOSRXFK7KHELRGHJUDGDEOHFRPSRQHQWVZLOOEHJLQWRGHWHULRUDWHXSRQH[SRVXUHWRDPELHQWFRQGLWLRQV
2EVHUYHVWHULOHWHFKQLTXHDWDOOWLPHVZKHQXVLQJWKH$QJLR6HDOGHYLFH
7KH$QJLR6HDOGHYLFHLVIRUVLQJOHXVHRQO\DQGVKRXOGQRWEHUHXVHGLQDQ\PDQQHU
7KH$QJLR6HDOGHYLFHPXVWEHLQVHUWHGWKURXJKWKHLQVHUWLRQVKHDWKSURYLGHGLQWKHNLW'RQRWVXEVWLWXWHDQ\RWKHUVKHDWK
8VHRQO\WKHDUWHULRWRP\ORFDWRUSURYLGHGLQWKHNLWWRORFDWHWKHSXQFWXUHLQWKHDUWHULDOZDOO
)ROORZSK\VLFLDQRUGHUVUHJDUGLQJSDWLHQWDPEXODWLRQDQGGLVFKDUJH
If the Angio-Seal device does not anchor in the artery due to improper orientation of the anchor or patient vascular anatomy, the absorbable components and delivery system should be
ZLWKGUDZQIURPWKHHPRVWDVLVFDQWKHQEHDFKLHYHGE\DSSO\LQJPDQXDOSUHVVXUH
If repuncture at the same location of previous Angio-Seal device use is necessary in
90 days , re-entry 1 cm proximal to the previous access site can be performed safely, based on
SXEOLVKHGPHGLFDOOLWHUDWXUH%HIRUHFRQVLGHULQJ$QJLR6HDOXVHDIHPRUDODQJLRJUDPRIWKHVLWHLVLQGLFDWHG>$SSOHJDWH55DQNLQ./LWWOH:.DKO).XWFKHU0
Restick following
initial Angioseal use
&DWKHWHUL]DWLRQDQG&DUGLRYDVFXODU,QWHUYHQWLRQV2FLDO-RXUQDORIWKH6RFLHW\IRU&DUGLDF$QJLRJUDSK\DQG,QWHUYHQWLRQV)HES@
³,ISDWLHQWVKDYHFOLQLFDOO\VLJQL¿FDQWSHULSKHUDOYDVFXODUGLVHDVHEDVHGRQSXEOLVKHGPHGLFDOOLWHUDWXUHWKH$QJLR6HDOGHYLFHFDQEHGHSOR\HGVDIHO\LQSDWLHQWDUWHULHV!PPGLDPHWHU
ZKHQWKHUHLVIRXQGWREHQROXPLQDOQDUURZLQJRIRUJUHDWHUZLWKLQPPRIWKHSXQFWXUHVLWH´$EDQGR$+RRG':HDYHU).DW]6
The use of the Angioseal device for
femoral artery closure.
-9DVF6XUJ
'LVSRVHRIFRQWDPLQDWHGGHYLFHFRPSRQHQWVDQGSDFNDJLQJPDWHULDOVXWLOL]LQJVWDQGDUGKRVSLWDOSURFHGXUHVDQGXQLYHUVDOSUHFDXWLRQVIRUELRKD]DUGRXVZDVWH