Part 1
Part 2
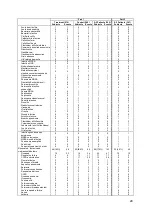
Treatment (270)
Control (280)
All Patients (550)
All Patients (347)
Subjects
Events
Subjects Events Subjects Events
Subjects
Events
Injury to liver
1
1
0
0
1
1
0
0
Liver disorder
0
0
1
1
1
1
0
0
Portal hypertension
0
0
1
1
1
1
0
0
Neoplasms benign, malignant and
unspecified (incl cysts and polyps)
5 (1.9%)
7
4 (1.4%)
5
9 (1.6%)
12
5 (1.4%)
5
Lung cancer
2
2
1
1
3
3
1
1
Large cell lung cancer
0
0
1
1
1
1
0
0
Lung nodule
0
0
1
1
1
1
0
0
Lymphocytic leukemia
0
0
1
1
1
1
0
0
Myelodysplastic syndrome
1
1
0
0
1
1
0
0
Ovarian cancer
1
1
0
0
1
1
0
0
Prostate cancer metastatic
0
0
1
1
1
1
0
0
Skin cancer
1
1
0
0
1
1
0
0
Small cell carcinoma of the lung
1
2
0
0
1
2
0
0
Adenoma
0
0
0
0
0
0
1
1
Breast cancer
0
0
0
0
0
0
1
1
Esophageal cancer
0
0
0
0
0
0
1
1
Lymphoma
0
0
0
0
0
0
1
1
Endocrine disorders
2 (0.7%)
2
1 (0.4%)
1
3 (0.5%)
3
1 (0.3%)
1
Adrenal insufficiency
1
1
0
0
1
1
0
0
Hypothyroidism
1
1
0
0
1
1
1
1
Myxedema
0
0
1
1
1
1
0
0
Immune system disorders
2 (0.7%)
2
1 (0.4%)
1
3 (0.5%)
3
0 (0.0%)
0
Amyloidosis
1
1
0
0
1
1
0
0
Heart transplant rejection
0
0
1
1
1
1
0
0
Transplant rejection
1
1
0
0
1
1
0
0
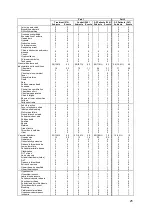
Skin and subcutaneous tissue
disorders
0 (0.0%)
0
3 (1.1%)
3
3 (0.5%)
3
3 (0.9%)
4
Diabetic ulcer
0
0
1
1
1
1
0
0
Foot ulcer
0
0
1
1
1
1
0
0
Venous stasis ulcer
0
0
1
1
1
1
0
0
Decubitus ulcer
0
0
0
0
0
0
1
1
Rash
0
0
0
0
0
0
1
2
Skin thinning of
0
0
0
0
0
0
1
1
Benign prostatic hypertrophy
0
0
1
1
1
1
0
0
Reproductive system and breast
disorders
0 (0.0%)
0
1 (0.4%)
1
1 (0.2%)
1
3 (0.9%)
3
Enlarged prostate
0
0
0
0
0
0
1
1
Postmenopausal bleeding
0
0
0
0
0
0
1
1
Vaginal bleeding
0
0
0
0
0
0
1
1
Adverse Device Events
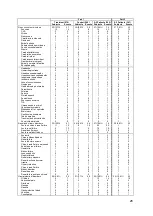
Table 16. Unanticipated or Serious Adverse Device Events Over Part 1 and Part 2
Part 1
Part 2
Treatment (270)
Control (280)
All Patients (550) All Patients (347)
Subjects Events Subjects
Events Subjects Events Subjects Events
Unanticipated
Serious Adverse
Device Events
0 (0.0%)
0
1 (0.4%)
1
1 (0.2%)
1
0 (0.0%)
0
Serious Adverse
Device Events
2 (0.7%)
2
0 (0.0%)
0
2 (0.4%)
2
0 (0.0%)
0
30