Page 38 of 48
Heatpipe Furnace Models 17702W, 17702P, 17702S (13
–
07/20)
THERMOMETRIC FIXED POINTS; A TUTORIAL
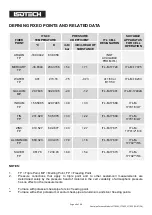
The International Temperature Scale, the scale most used in science and industry, is based on a series of
fixed point temperatures. Fixed points involve two-phase or three-phase equilibria of, ideally, pure
materials to which constant temperature values have been assigned by primary thermometry. Defining
fixed points are chosen to be as few in number as is consistent with the need to establish satisfactory
interpolation procedures.
There are secondary reference points which, also, are two-phase or three-phase equilibria of very pure
materials, whose temperature values have been established by measurements made with interpolation
instruments calibrated at the defining fixed points. Secondary reference points are useful for the calibration
of thermometers having total ranges shorter than the interpolation ranges of the Scale. Generally,
secondary points are admitted to the Scale only if the material is generally available in high purity and if
sufficient reproducibility of the equilibrium temperature has been confirmed by measurements made
independently by a considerable number of investigators. An average value (weighted according to
individual uncertainties) is then used as the ITS temperature value.
Two-phase equilibria may be solid-liquid, liquid-vapour, or solid-vapour. From the Phase Rule of Gibbs:
P + F = C + 2
P is an integer equal to the number of phases present, C is the number of components present - for a pure
material C = 1 - and F is an integer representing the number of degrees of freedom. It is evident that the
temperatures of two-phase equilibria are pressure-dependent (one degree of freedom only) whereas
equilibria in which all three phases are present (triple points) are characterised by unique values of
temperature and pressure (zero degrees of freedom).
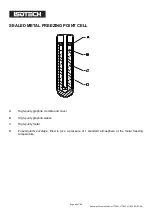
A necessary condition to establish a triple point is to contain the appropriate material in a sealed enclosure
from which all other materials, including air, have been evacuated, leaving a space to be filled by the
vapour phase at a pressure appropriate to the temperature. When the three-phase (solid, liquid, vapour)
condition has been established, these parameters will settle to their unique triple-point values.
The defining fixed points above 0°C are liquid-solid equilibria of high-purity metals. Pressure-dependence
is small and thermal capacity and thermal conductivity are relatively high. Metals are generally available
with a purity of 99.999% ("five-nines") or 99.9999% ("six-nines").
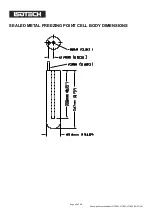
The metal is contained in a crucible of purified graphite, with a graphite cover and a graphite re-entrant
sleeve. The crucible is enclosed in an envelope of fused quartz, which extends into the sleeve interior to
form the thermometer well. The cell is charged with a pure metal, purged and filled with sufficient argon (or
another inert gas) to give a pressure of 101kPa (1 standard atmosphere) at the freezing temperature and
then sealed. Thus, it is at once protected from contamination and supplied with an inert atmosphere at the
required pressure at the equilibrium temperature. A correction for the effect of change in ambient pressure
on freezing point need not be made. Sealed cells of this type have shown no measurable change after 15
years of use.
In general, sealed fixed-point cells are used in vertical-tube furnaces which provide good temperature
control and sufficient cell immersion to make axial temperature gradients, in the measurement zone,
negligible. With the cell in the furnace, the controller is first set about 5°C above the anticipated value
corresponding to the melting temperature of the metal in the cell. The onset of melting is indicated by a
cessation of temperature rise because of the latent heat required to produce the phase change. This melt
plateau can last for a considerable period of time. When melting is complete, the cell temperature will rise
to the furnace temperature.