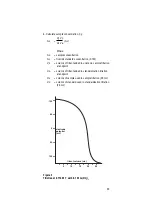
Limits of Detection
In neutral solutions, fluoride concentration can be measured
down to 10
-6
M (0.02 ppm) fluoride. However, care must be
taken in making determinations below 10
-5
M to avoid sample
contamination. The upper limit of detection is a saturated
fluoride solution.
Temperature Effects
Since electrode potentials are affected by changes in
temperature, samples, and standard solutions should be within
± 1 °C (± 2 °F) of each other. At the 10
-3
M level, a 1 °C
difference in temperature results in a 2% error. The absolute
potential of the reference electrode changes slowly with
temperature because of the solubility equilibria on which the
electrode depends. The slope of the fluoride electrode also
varies with temperature, as indicated by the factor “S” in the
Nernst equation Values of the Nernst factor for fluoride ion are
given in
Table 5.
If temperature changes, meter and electrodes
should be recalibrated.
The electrode can be used at temperatures from 0 to 100 °C,
provided that temperature equilibrium has occurred. For use at
temperatures substantially different from room temperature,
equilibrium times of up to one hour are recommended. The
electrode must be used only intermittently at solution
temperatures above 80 °C.
Table 5
Values of Theoretical Slope vs. Temperature
Temperature (°C)
Slope (mV)
0
- 54.2
10
- 56.2
20
- 58.2
25
- 59.2
30
- 60.1
40
- 62.1
50
- 64.1
34
Summary of Contents for 96-09
Page 7: ...4 ...