13
〔
Features of Niobium Rechargeable Lithium Batteries
〕
Stable Operating Voltage of 1.2V
With a discharge voltage of 1.2V, Sanyo niobium rechargeable lithium
batteries maintain stable voltage characteristics over a prolonged period.
Charge/Discharge Characteristics
The NBL621 can withstand 3,000 cycles under discharge
conditions of 0.2mAh (discharge depth of 5%)
Wide Operating Temperature Range
The use of an organic electrolyte gives an extended operating
temperature range:
−
20
℃
to
+
60
℃
.
Charging Possible with 1.8V
Even a charging voltage as low as 1.8V ensures high charge efficiency.
Small Self-Discharge Rate Assures Durability
The self-discharge rate of approx. 2% per year at room
temperature is much lower than that of Ni-Cd button cells. Even
after five years of storage at room temperature, you can expect
about 90% of the original capacity.
Greater Safety
(UL approved)
Since Sanyo niobium rechargeable lithium batteries do not
contain toxic materials, noxious liquids or gases, they pose no
major pollution problems. They are UL recognized components
(File No. MH12383).
Sanyo niobium rechargeable lithium batteries are high-capacity coin-type batteries that can be recharged
at 2.0V. These batteries are specially designed for use with low-voltage equipment. Similarly with the ML-
series manganese dioxide rechargeable lithium batteries, the NBL-series features a low self-discharge
rate, thus offering an expected life of 5 years at room temperature. These batteries are optimized for use
as memory backup power sources and can also be used in combination with solar cells.
1.0
1.5
2.0
2.5
0
100
200
300
400
〜
discharge time in hours
load : 100kΩ(=13μA)
temp: 23℃
for shipment
fully charged
cell voltage (V)
●
Discharge
NBL621
〔
Principles and Structure of Coin Type Niobium Rechargeable Lithium Batteries
〕
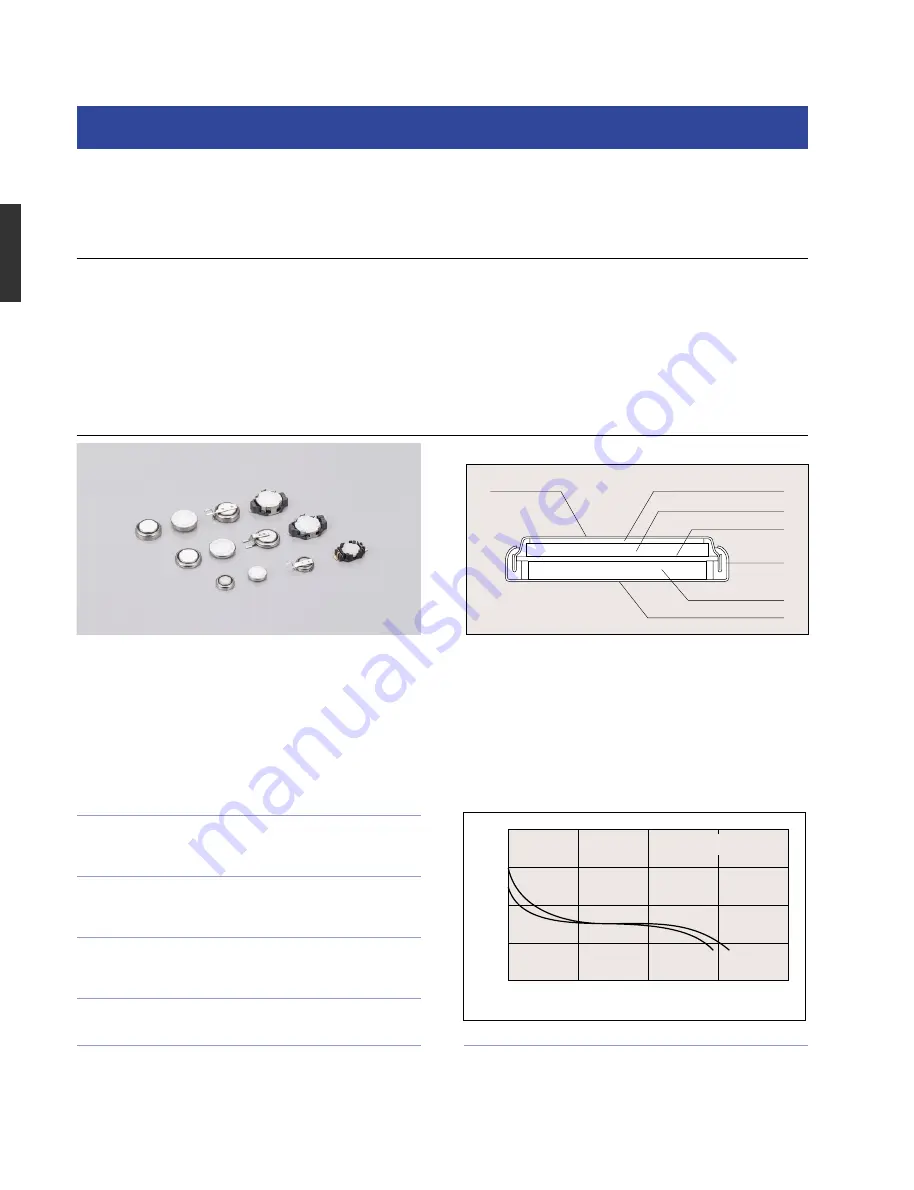
Niobium Rechargeable Lithium Batteries (NBL series)
These batteries use niobium pentoxide as the active material for the positive electrode by employing Sanyo's original treatment process. In
turn, the active material for the negative electrode is made from lithium aluminum alloy. This allows stable cyclic performance to be
maintained at a low discharge voltage.The electrolyte is made by dissolving lithium salts in a mixed organic solvent.
The charge/discharge reaction of the batteries is as follows:
●
Anode reaction: (Li
−
A
R
)
→
←
A
R
+
Li
+
+
e
−
●
Cathode reaction: Nb
Ⅴ
2
O
5
+2
Li
+
+2
e
−
→
←
Nb
Ⅳ
2
O
5
(2Li
+
)
●
Overall battery reaction: Nb
Ⅴ
2
O
5
+2
(Li
−
A
R
)
→
←
Nb
Ⅳ
2
O
5
(2Li
+
)
+2
A
R
The cell voltage is approximately 2V.
■
Battery Structure
■
When the batteries are used with a contact system
●
Use nickel-plated phosphor bronze or stainless steel for
terminal materials to make contact with the batteries.
●
For stable contact conditions, several N of contact pressure
are recommended for the contact.
■
Applications
●
Memory backup power sources for pagers and portable
cellular phones including PHS.
●
Power source for portable equipment.
●
Hybrid power source when combined with solar cells
●
Memory backup power source for electronic equipment.
;;
;;;
;;
;;;;;;;;;;;;;;;;;;;;;;;
;;;;;;;;;;;;;;;;;;;;;;;
;;;;;;;;;;;;;;;;;;;;;;;
;;;;;;;;;;;;;;;;;;;;;;;
negative current collector
cathode (Nb
2
O
5
)
positive can
negative cap
anode (Li−A
R
)
gasket
separator+electrolyte
Rechargeable
Lithium
Batteries
















