5
Figure 4.
TriVascular Fill Polymer Kit
1.3. Ancillary Components
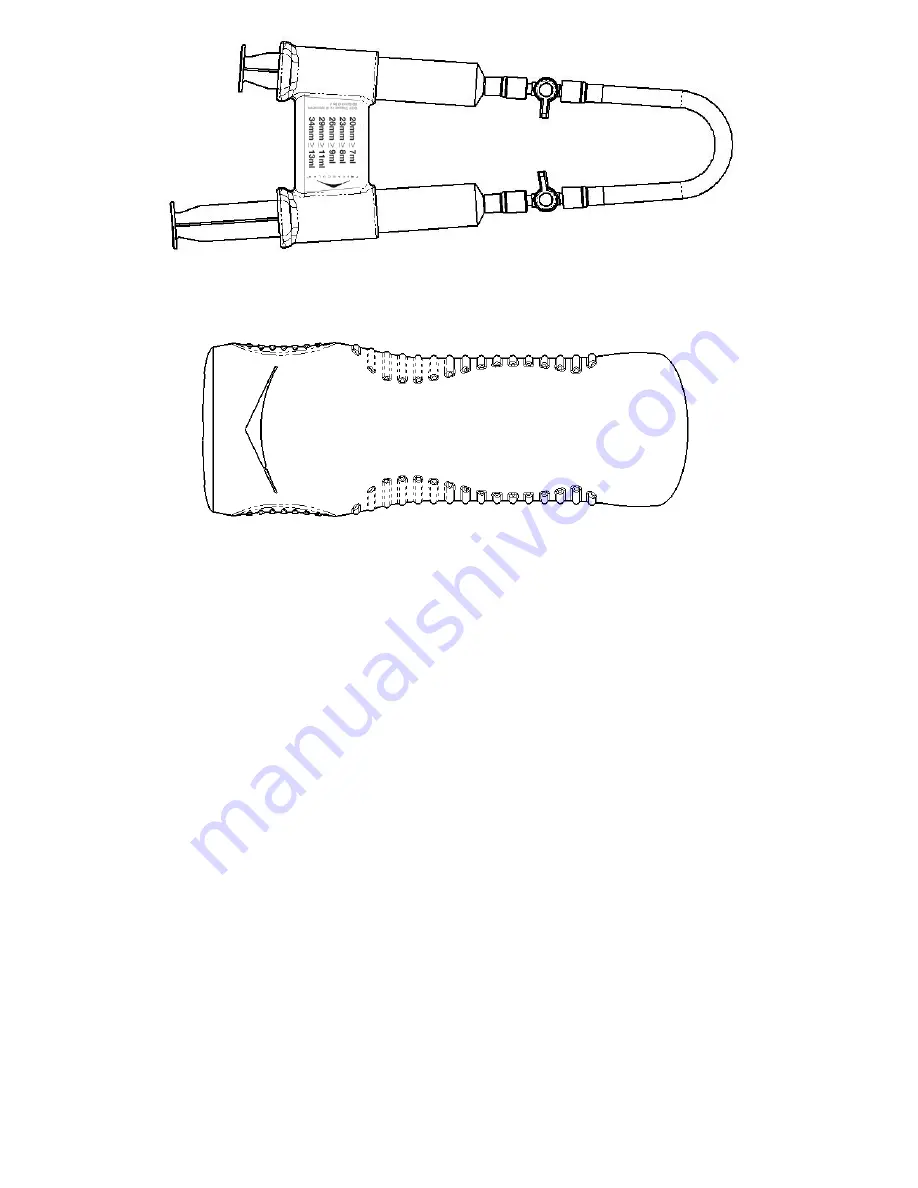
Figure 5.
TriVascular Autoinjector
2.
Indications for Use
The TriVascular Ovation Abdominal Stent Graft System is indicated in subjects
diagnosed with an aneurysm in the abdominal aorta having vascular morphology
suitable for endovascular repair, including:
•
Adequate iliac/femoral access compatible with vascular access techniques,
devices, and/or accessories,
•
Non-aneurysmal proximal aortic neck:
-
with a length of at least 7 mm proximal to the aneurysm,
-
with an inner wall diameter of no less than 16 mm and no greater than 30
mm, and
-
with an aortic angle of
≤
60 degrees if proximal neck is
≥
10 mm and
≤
45
degrees if proximal neck is < 10 mm,
•
Non-aneurysmal distal iliac landing zone:
-
with a length of at least 10 mm,
-
with an inner wall diameter of no less than 8 mm and no greater than 20
mm.
3.
Contraindications
•
Patients who have a condition that threatens to infect the graft.
•
Patients with sensitivities or allergies to the device materials.




















