5
4
Chapter 5 : CONSTRUCTION
7. Electronic monitoring equipment (such as ECG monitors and ECG alarms)
may not operate properly when EMS stimulation is in use.
8. There should be a prominently placed statement warning that stimulus
delivered by this device may be sufficient to cause electrocution. Electrical
current of this magnitude must not flow through the throax because it may
cause a cardiac arrhythmia.
9. Do not place electrodes on the front of the throat as spasm of the Laryngeal
and Pharyngeal muscle may occur.
10. Care should be taken so that when operating potentially dangerous ma-
chinery the stimulator controls are not changed abruptly.
6. Electrodes should not be placed over the eyes, in the mouth, or internally.
11. Keep this device out of the reach of children.
12. Caution: Federal law restricts this device to sale by or on the order of a
physician.
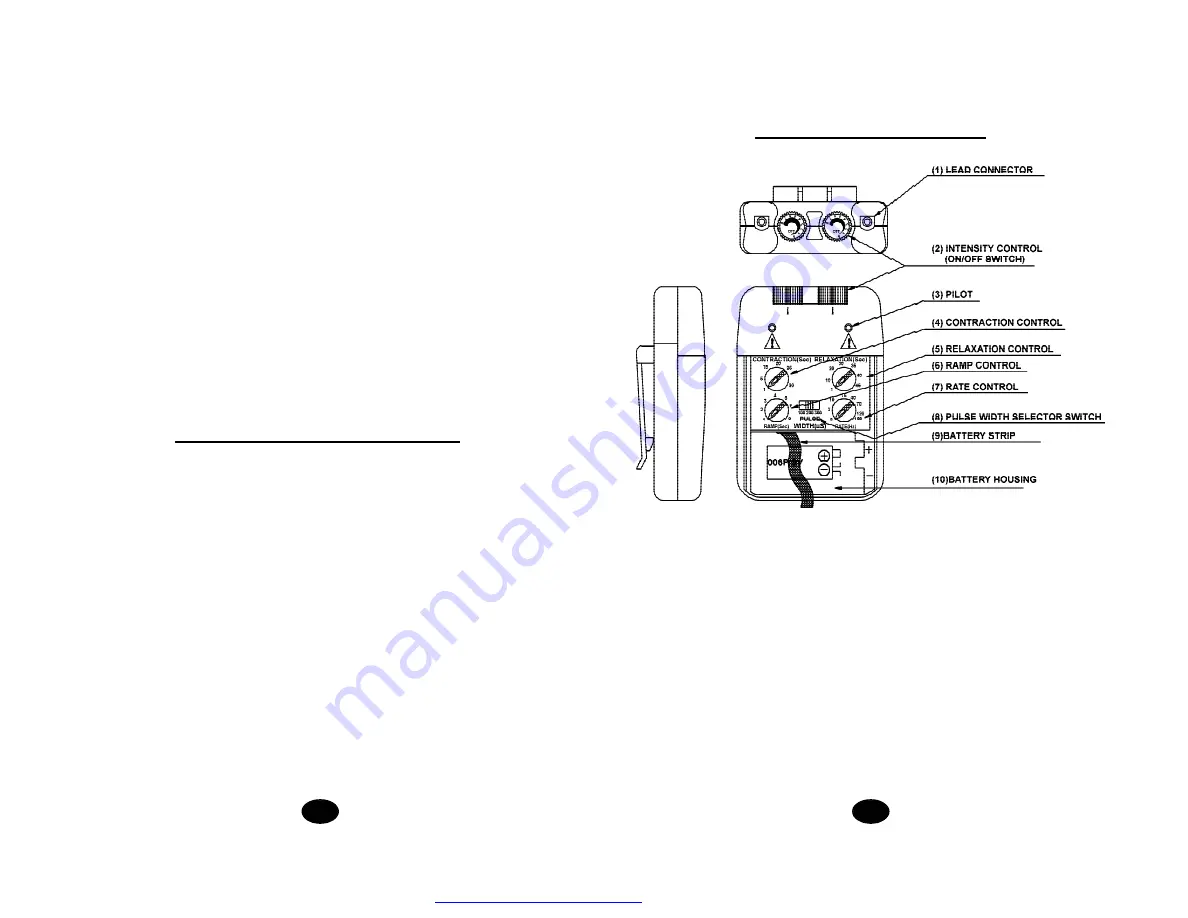
Chapter 4 : GENERAL DESCRIPTION
The AMS-4 EMS is a battery operated pulse generator that sends electrical
impulses through electrodes to the body and reaches the underlying nerves or
muscle group. The device is provided with two controllable output channels,
each independent of each other. An electrode pair can be connected to each
output channel.
The electronics of the AMS-4 EMS create electrical impulses whose Intensity,
Pulse Width, Pulse Rate, Contraction, Relaxation and Ramp may be altered
with the switches. Dial controls are very easy to use and the slide cover pre-
vents accidental changes in the setting.
PDF created with FinePrint pdfFactory trial version































