The mean follow-up during Randomized Access was 17.6 months for a total duration of
approximately 800 patient years. During the course of Randomized Access, 93 patients
in the Treatment group and 110 patients in the Control group exited the study with the
primary reason being death.
A total of 347 patients (177 in the Treatment group and 170 in the Control group)
completed Randomized Access and entered Open Access. The mean follow-up during
Open Access was 13 months for a total duration of approximately 400 patient years.
During the course of Open Access, 58 patients in the Treatment group and 43 patients in
the Control group exited the study with the primary reason being death.
Primary and Secondary Endpoint Results
Primary Safety Endpoints
The CHAMPION clinical trial met the two primary safety endpoints: (1) Freedom from
device/system related complications (DSRC) and (2) Freedom from sensor failure. The
protocol pre-specified objective performance criteria (OPC) were that at least 80% of
patients were to be free from DSRC and at least 90% were to be free from pressure
sensor failure. Of the 575 patients in the safety population, 567 (98.6%) were free from
DSRC at 6 months (lower confidence limit 97.3%, p<0.0001). This lower limit of 97.3% is
greater than the pre-specified OPC of 80% (Table 6a and 6b). There were no sensor
explants or repeat implants and all sensors were operational at 6 months for a freedom
from sensor failure of 100% (lower confidence limit 99.3%, p<0.0001). This lower limit of
99.3% is greater than the pre-specified OPC of 90% (Table 7).
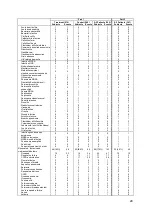
Table 6a. Primary Safety Endpoint – Freedom from Device/System Related Complications
Device/System Related
Complications (n=575)
Lower 95.2%
Confidence
Limit
2
Objective Performance
Criterion (OPC)
p-value
3
Yes
No
8 (1.4%)
1
567 (98.6%)
97.3%
80%
p<0.0001
1
DSRCs (8 total) by group: Consented but not randomized (2), Treatment (3), Control (3)
2
Exact 95.2% Clopper-Pearson lower confidence limit
3
p-value from exact test of binomial proportions compared to 80% for all patients
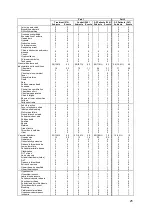
Table 6b. Primary Safety Endpoint – Description of Device/System Related Complications
Description
Number of Subjects with Device or System
related complication (%) (N = 575)
Hemoptysis
1 (0.2%)
Sensor did not deploy
1 (0.2%)
Transient Ischemic Attack (TIA)
1 (0.2%)
Atypical chest pain
1 (0.2%)
Sepsis → death
1 (0.2%)
Atrial arrhythmia → death
1 (0.2%)
Arterial embolism (upper extremity)
1 (0.2%)
Pulmonary artery (in-situ) thrombus
1 (0.2%)
Total Subjects Experiencing a DSRC
8 (1.4%
[1]
, 95.2% LCB 97.3%)
15