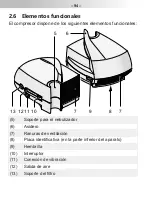
6.4 Information about electromagnetic
compatibility
Electrical medical equipment is subject to special precautionary
measures with regard to electromagnetic compatibility (EMC).
Such equipment must only be installed and operated in accord-
ance with EMC instructions.
Portable and mobile high-frequency communication devices
can disrupt electrical medical equipment. Using accessories,
converters and power cords other than those specified (with
the exception of converters and power cords that the manufac-
turer of the medical electrical device sells as spare parts for in-
ternal components) can result in higher emission levels or
lower the resistance to interference of the device.
The device must not be placed directly beside or on top of
other devices for operation. If the medical electrical device
must be placed beside or on top of other devices to operate it,
it should be monitored constantly to ensure that it is operating
properly in the arrangement used.
Technical data on electromagnetic compatibility (EMC informa-
tion) is available in table format upon request from PARI GmbH
or on the internet at the following linked page:
https://www.pari.com/fileadmin/Electromagnetic-compatibility-1.pdf
6.5 Ambient conditions
Operation
Ambient temperature
+10 °C to +30 °C
Relative humidity
30% to 75% (non-condensing)
Atmospheric pressure
700 hPa to 1,060 hPa
Use of the device in professional healthcare facilities is limited
to the inpatient wards and the intensive care unit. Use of the
– 80 –