IFU 12296 Rev C
Invuity Customer Service: 866.711.7768
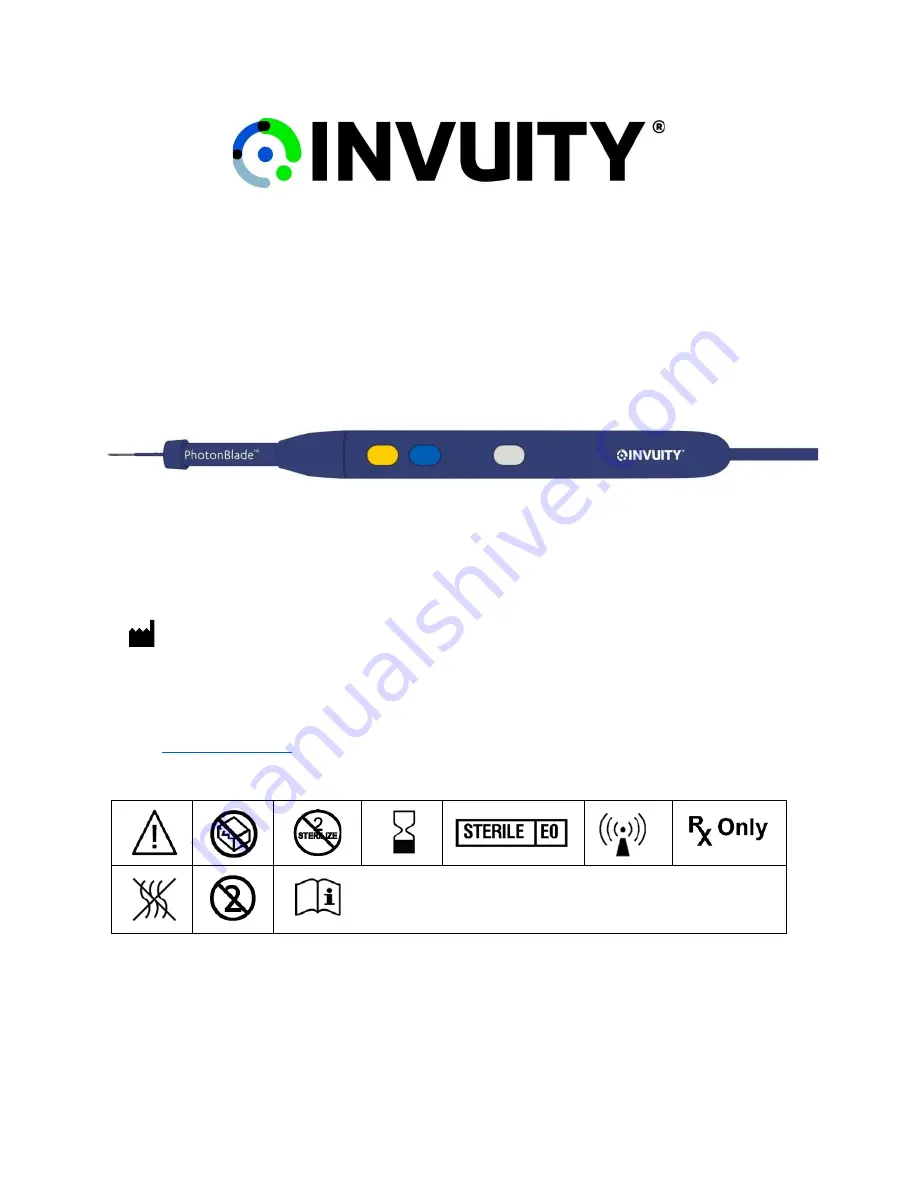
PhotonBlade
®
Instructions for Use
MANUFACTURER
Invuity, Inc.
444 De Haro Street
San Francisco, CA 94107 USA
Tel: 866.711.7768
www.invuity.com
Consult Instructions for Use and Symbols Glossary on
this website: www.invuity.com/documentlibrary