47
Section 4
Platinum Series pH
Electrode Characteristics
4.1 Theory of Operation
pH is a measure of the hydrogen ion activity in a solution and
is defined as: -log
10
a
H+
where a
H+
is the activity of the
hydrogen ion. The 0-14 range of pH measurement is the
measurement of a difference in hydrogen ion concentration of
100,000,000,000,000 (1 x 10
14
). This means that at pH 0, the
hydrogen ion concentration is 1 x 10
14
times greater than at pH
14. This also means that the hydroxyl ion concentration at pH
14 is 1 x 10
14
times greater than at pH 0.
When the hydrogen and hydroxyl ions are present in equal
numbers (the neutral point), the pH is 7. pH values from 0 to 7
are termed acidic and those from 7 to 14 are termed basic. Note
that a pH change of one unit (for instance from pH 6 to pH 7)
is a factor-of-10 change in hydrogen ion concentration.
The glass membrane of a pH electrode responds to the
hydrogen ion activity by developing an electrical potential at
the glass/liquid interface. At a constant temperature, this
potential varies linearly with the pH of the solution being
measured. The change in potential per pH unit is the slope of
the electrode. The slope of the electrode increases linearly with
temperature.
The potential inside the pH glass bulb is fixed by the filling
solution, and the reference electrode potential is constant. For
these reasons, any change in the potential of the electrode
system at a given temperature will be due to any change in the
pH of the solution being measured.
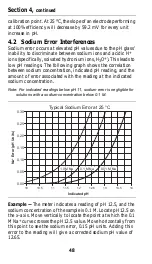
Effects of temperature on pH measurements depend on the
reference electrode used, pH of the solution within the pH
electrode, and pH of the test solution. At a certain pH,
temperature will have no effect on the potential of the electrode
system. This is known as the isopotential point. Also, at some
pH level, the system will exhibit no potential. This is known as
the zero potential point. Both the isopotential point and the
zero potential point are features designed into electrodes. Hach
electrodes are designed so the isopotential and zero potential
points are at pH 7 to minimize temperature effects at this
Содержание PLATINUM Series
Страница 2: ...2...
Страница 4: ...4...
Страница 6: ...6...
Страница 8: ...8...
Страница 16: ...16...
Страница 17: ...17 Section 2 Applications...
Страница 18: ...18...
Страница 26: ...26...
Страница 42: ...42...
Страница 46: ...46...
Страница 52: ...52...
Страница 54: ...54...
Страница 60: ...60...
Страница 64: ......