Monnal T75 user manual
9 Maintenance
Accessories can be re-usable (autoclavable) or single-use (disposable).
Re-usable elements must be regularly cleaned and sterilized to prevent infection.
This procedure, which is mandatory and extremely important, is the responsibility of the user.
9.1 Definitions
Cleaning
The act of removing all traces of soiling from a place, a surface, or an element.
Sterilization
Total destruction of all germ strains, viruses, and yeasts.
A sterilization or disinfection procedure is never possible on dirty or soiled elements.
A complete procedure includes:
1.
Disassembly, pre-disinfection, rinsing and drying;
2.
Cleaning, rinsing and drying;
3.
Disinfection, rinsing and drying, or sterilization;
4.
Reassembly and functional tests
CAUTION:
Never use abrasive powders, acetone, or other powerful solvents.
Note:
The following instructions have been approved by the manufacturer of the medical
equipment as ENABLING a medical device to be prepared for re-use.
It is always the responsibility of the department concerned to ensure that the sterilization procedure
actually implemented via the equipment, materials and personnel of the reprocessing facility achieves
the expected result.
This generally requires the validation and routine inspection of procedures.
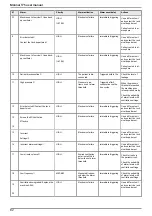
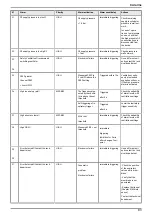
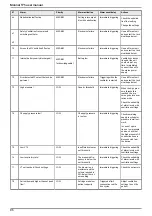
9.2 Routine maintenance
The surface of the ventilator can be cleaned.
Air Liquide Medical Systems recommends the following cleaning products for this purpose: ANIOS
TSA or ANIOS SURFA’safe.
Obey the instructions of the product manufacturer, and do not allow any liquid to penetrate inside the
unit.
CAUTION:
Maintenance must be carried out with the ventilator disconnected from the elec-
trical power supply.
92