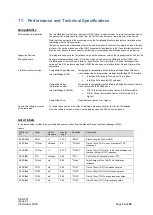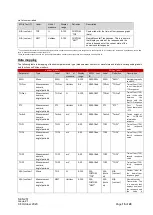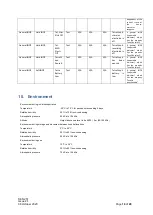
SEN 257
Issue 2.1
06 October 2020
Page
2
of
29
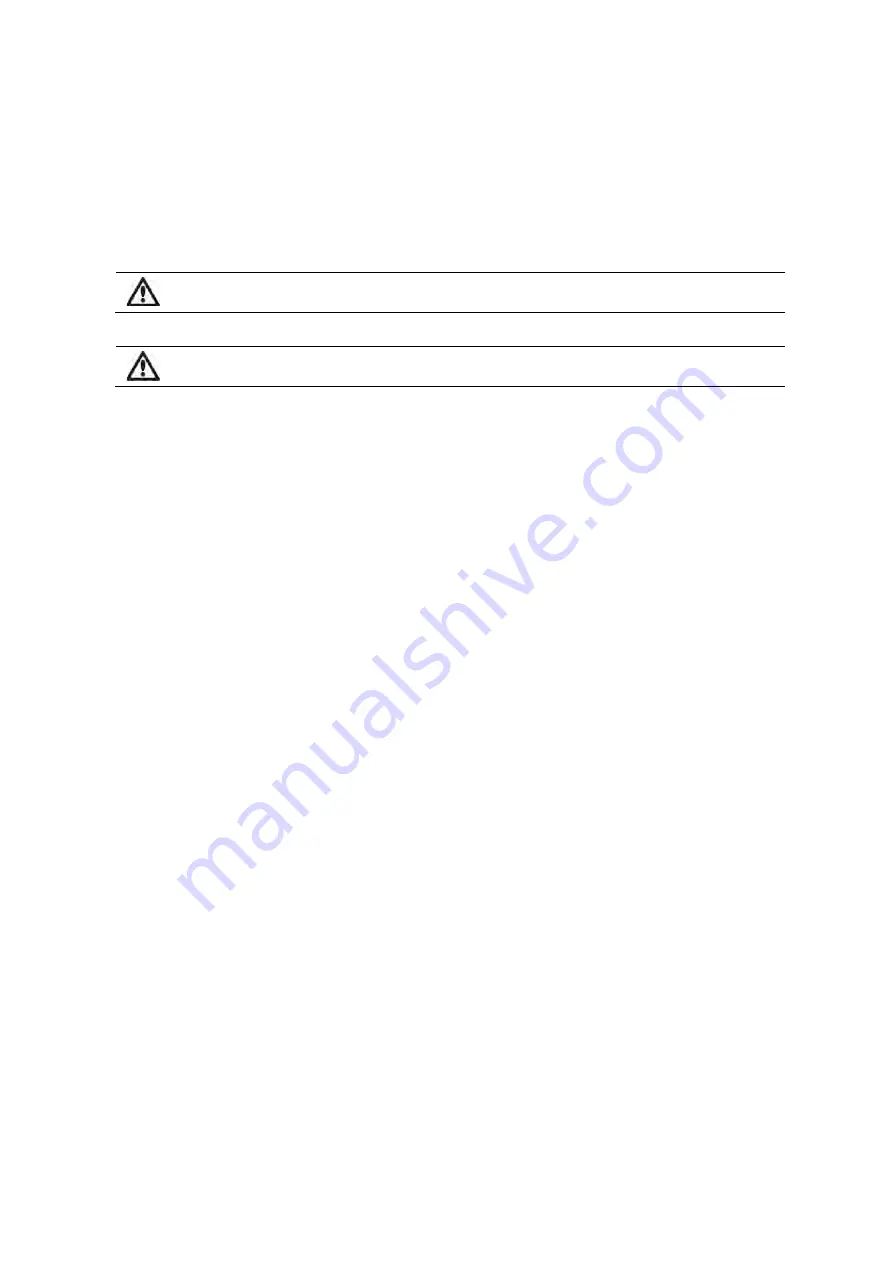
Figure 1
. Description of a warning and a caution.
3. Warnings and cautions
The European Medical Device Directive requires all manufacturers to include appropriate
warnings and cautions (Figure 1) for their equipment and many of the warnings and
cautions shown here also apply to similar devices.
To make sure that all users are well informed, various warnings and cautions are made
throughout these instructions.
A WARNING
is given when the personal safety of the patient or user may be affected and when
disregarding this information could result in injury.
A CAUTION
is given when special instructions must be followed. Disregarding this information could cause
damage to the device.
4. Scope of Use and Contraindications
The intended use of the TetraGraph Philips interface device is to connect a TetraGraph
Monitor(SEN 2001) to a Philips IVOI compatible monitor so that the Neuromuscular
Transmission data; TOF Ratio and TOF Count, PTC and ST measurements monitored by the
TetraGraph can be displayed on the IVOI enabled monitor.
Indications for use is:
To connect the TetraGraph to a Philips IntelliVue (IVOI) Monitors to allow data transfer and
the display of TetraGraph data on the Philips monitor.
Contraindications
No contraindications have been identified for the intended use of the TetraGraph Philips
Interface.
5. Intended Users
The intended user of the TetraGraph Philips Interface product is the same user group as
intended for the TetraGraph Monitor and the Philips IntelliVue Monitor.