16 Probes and Biopsy
Operator’s Manual
16 - 11
16.1.5 Probes Cleaning and Disinfection/Sterilization
Before and after completing each examination, clean and disinfect (or sterilize) the probes as
required. When biopsy procedures have been performed, be sure to sterilize the needle-guided
bracket. Fail to do so may result in the probe and the needle-guided bracket to becoming sources of
infection. Please follow the instructions in the manual for cleaning.
WARNING
Never immerse the probe connector into liquid such as water or disinfectant.
Immersion may cause electrical shock or malfunction.
CAUTION
•
No cleaning and disinfecting may result in the probe becoming a source of
infection.
•
Please follow the disinfectant manufacturer’s manual for performing
cleaning and disinfection, including preparing sterile water and cleaning
and disinfection time.
NOTE:
•
After the examination, wipe off the ultrasound gel thoroughly. Otherwise, the ultrasound gel
may solidify and degrade the image quality of the probe.
•
DO NOT make the probe to become overheated (more than 55 °C) during cleaning and
disinfections. High temperature may cause the probe to become deformed or damaged.
•
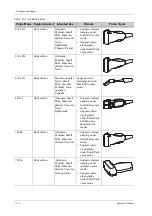
Observe the graph here carefully to immerse the probe. Only soak parts of the probe below the
strain relief.
•
Repeated disinfection will eventually damage the probe, please check the probe performance
periodically.
Cleaning and Disinfection/Sterilization Overview
Cleaning and disinfection refer to two distinct processes. According to the Centers for Disease
Control and Prevention (CDC) “Guideline for Disinfection and Sterilization in Healthcare
Facilities” (2008):
•
Cleaning is the removal of visible soil (e.g. organic and inorganic material) from objects and
surfaces and normally is accomplished manually or mechanically using water with detergents
or enzymatic products. Thorough cleaning is essential before high-level disinfection and
sterilization because inorganic and organic material that remains on the surfaces of instruments
interfere with the effectiveness of these processes.
•
Disinfection describes a process that eliminates many or all pathogenic microorganisms,
except bacterial spores.
–
Low-Level Disinfection—Destruction of most bacteria, some viruses, and some fungi.
Low-level disinfection will not necessarily inactivate Mycobacterium tuberculosis or
bacterial spores.
–
High-Level Disinfection (HLD)—Destruction/removal of all microorganisms except
bacterial spores.
Summary of Contents for Ana
Page 2: ......
Page 50: ...This page intentionally left blank...
Page 60: ...This page intentionally left blank...
Page 110: ...This page intentionally left blank...
Page 116: ...This page intentionally left blank...
Page 166: ...This page intentionally left blank...
Page 176: ...This page intentionally left blank...
Page 194: ...This page intentionally left blank...
Page 220: ...This page intentionally left blank...
Page 288: ...This page intentionally left blank...
Page 304: ...This page intentionally left blank...
Page 308: ...This page intentionally left blank...
Page 316: ...This page intentionally left blank...
Page 337: ......
Page 338: ...P N 046 018835 00 2 0...